Malignant syphilis (MS) is a rare manifestation of secondary syphilis which mainly occurs in immunocompromised individuals such as those coinfected with human immunodeficiency virus (HIV). However, recent reports have described MS in immunocompetent individuals. To describe the characteristics of individuals with MS and associated risk factors, a review of case reports published from 2014 to 2018 was conducted. Out of 45 published case reports, 33 cases (73%) occurred in HIV-positive individuals with majority having CD4 counts <500 cells/mm3. Of the 12 cases (27%) in HIV-negative individuals, half had comorbidities such as diabetes mellitus, alcoholism, drug abuse, psoriasis, and hepatitis. The most frequent manifestation of MS was ulceronodular cutaneous lesions with central adherent crust, which affected the face, trunk, and limbs. Given the increasing number of MS regardless of the immune status, dermatologists and general practitioners should be vigilant to allow early diagnosis and treatment, hence reducing their morbidity.
La sífilis maligna (SM) es una manifestación poco común de la sífilis secundaria. Esta se presentará principalmente en individuos inmunodeprimidos, como es el caso de los pacientes con una coinfección por el virus de la inmunodeficiencia humana (VIH). Sin embargo, recientemente se han descrito casos de SM también en individuos inmunocompetentes. Se realizó una revisión de los casos publicados entre el año 2014 y el 2018 para recoger las características de los pacientes con SM, así como los factores de riesgo asociados. De los 45 casos publicados, 33 casos (73%) ocurrieron en personas VIH positivas, la mayoría con recuentos de CD4 < 500 células/mm3. De los 12 casos (27%) en pacientes VIH-negativo, la mitad tenía comorbilidades como diabetes mellitus, alcoholismo, abuso de drogas, psoriasis y hepatitis. La manifestación más frecuente de la SM fueron las lesiones cutáneas ulcero – nodulares, las que presentaban una costra central adherente, y que afectaban la cara, el tronco y las extremidades. Dado el creciente número de SM, independientemente del estado inmunológico, los dermatólogos y médicos generales deben tener en cuenta la existencia de esta entidad para así poder realizar un diagnóstico y tratamiento oportuno, reduciendo de esta manera la morbilidad asociada.
Syphilis is an infection caused by Treponema pallidum, a spirochete bacterium that exclusively infects humans.1 Malignant syphilis (MS), also known as syphilis maligna, lues maligna, or rupioid syphilis, is a rare aggressive form of secondary syphilis.1 The term “rupioid” stems from the rupia or “oyster-like” appearance of these lesions, describing well-demarcated plaques with thick, lamellate, and adherent crusts on the surface that resemble an oyster shell.2 In 1859, the French dermatologist Pierre Bazin first used the term malignant to describe a case of secondary syphilis, and in 1896, at the Third International Congress of Dermatology in London, the Danish dermatologist Haslund and German dermatologist Neisser, independently classified MS as a rare, aggressive, ulcerating form of secondary syphilis and not an early form of tertiary syphilis, as had previously been assumed.1,3 It is distinguished from classical secondary syphilis based on a more severe general clinical picture and the presence of pleomorphic and ulceronecrotic skin lesions.4
MS presents as crusted or scaly papules and plaques that can ulcerate or become necrotic.5 The exanthem mainly affects the trunk and limbs, although the face, scalp, mucous membranes, palms, and soles can also be affected. Palpable peripheral lymphadenopathy, fever, and constitutional symptoms are commonly observed in patients with the disease. There have been only a limited number of reports of MS published in medical literature, and most of the reported cases have been in immunocompromised individuals, particularly those infected with human immunodeficiency virus (HIV).6 Syphilis and HIV co-infection can lead to an increased HIV viral load, concomitantly with a decreased CD4+ cell counts, greatly increasing the risk of MS, especially if untreated.7,8 Before the HIV pandemic, MS was extremely rare; between 1900 and 1988, only 14 cases had been published in English language. At present, it is estimated that up to 7% of all syphilis cases in immunocompromised patients meet the criteria for MS, often presenting as the first clinical manifestation of HIV infection.4 However, some reports on MS in immunocompetent individuals have been recently published.9,10 We conducted a systematic review of case reports on MS published between 2014 and 2018 to analyze demographic and risk factors of MS.
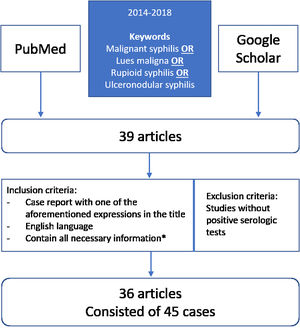
MethodsIn order to review clinical, laboratory, and therapeutic data from patients with documented MS, we searched 2 databases (PubMed and Google Scholar) for literature published between 2014 and 2018, using the following terms: malignant syphilis OR lues maligna OR rupioid syphilis OR ulceronodular syphilis (Fig. 1). Our literature search yielded 39 articles, which were independently reviewed by 2 investigators, and any disagreement was resolved by consensus. We included all studies where the diagnosis was documented by serology or any combination of serology, pathologic findings, and immunostaining. Studies without positive serologic tests were not included. The inclusion criteria for the articles were: (1) case report with one of the aforementioned expressions in the title; (2) article in English; (3) articles published between 2014 and 2018; and (4) contain all the necessary information (gender, age, sexual behavior (heterosexual, bisexual or homosexual), location and description of the lesions, onset, serology and histopathology results, HIV status, immunostaining, treatment and post-treatment reactions, outcome). In total, 36 of the 39 articles were considered relevant and met the inclusion criteria. From those selected articles, we collected a total of 45 cases of MS.2,3,9–39 We extracted the details on each case and recorded it on a spreadsheet Microsoft Excel 2016. We used Microsoft Excel 2016 to produce basic descriptive statistics (frequencies, percentages, and medians) of the data. We did not do any hypothesis testing.
ResultsDemographic characteristicsThe majority (84%) of the patients were male, and the patients had a median age of 41 years (range: 20–86 years). The highest incidence was in the 40-44 years age group (Table 1). There was minimal information available regarding high-risk behaviors (possibly related to syphilis acquisition) in the cases we reviewed.
Características demográficas y los factores de riesgo de sífilis en pacientes con sífilis maligna.
| Characteristics | Patients (n) | (%) |
|---|---|---|
| Sex | ||
| ○ Male | 38 | 84.4 |
| ○ Female | 7 | 15.6 |
| Age group | ||
| ○ 20-24 | 3 | 6.7 |
| ○ 25-29 | 7 | 15.6 |
| ○ 30-34 | 1 | 2.2 |
| ○ 35-39 | 9 | 20 |
| ○ 40-44 | 13 | 28.9 |
| ○ 45-54 | 8 | 17.8 |
| ○ 55-64 | 2 | 4.4 |
| ○ >65 | 2 | 4.4 |
| Positive serology | ||
| Non-treponemal (VDRL, RPR) | 41† | – |
| Treponemal (FTA-ABS, TPHA, TPPA) | 35† | – |
| HIV status | ||
| ○ Positive | 33 | 73.3 |
| ○ Negative | 12 | 26.7 |
| HIV (+) CD4 count (cells/mm3) | ||
| ○ <200 | 8 | 24.2 |
| ○ 200-499 | 17 | 51.5 |
| ○ >500 | 5 | 15.2 |
| ○ Not mentioned | 3 | 9.1 |
| Treatment | ||
| ○ BPG | 29 | 64.4 |
| ○ Penicillin G | 7 | 15.6 |
| ○ Doxycycline | 6 | 13.3 |
| ○ Ceftriaxone | 3 | 6.7 |
| JHR | ||
| ○ Positive | 9 | 20 |
| ○ Negative | 36 | 80 |
BPG: Benzathine Penicillin G; JHR: Jarisch-Herxheimer Reaction; VDRL: Venereal Disease Research Laboratory; RPR: Rapid Plasma Reagin; FTA-ABS: Fluorescent Treponemal Antibody Absorption; TPHA: Treponema Pallidum Hemagglutination Assay.
The clinical manifestations are reported on Table 2. The most frequent clinical manifestations were ulceronodular cutaneous lesions with adherent crust on the central surface affecting the face, trunk, and/or limbs (Table 2). Serological data were available for 44 out of 45 cases. The syphilis diagnosis was confirmed by nontreponemal and treponemal tests, which were reported as positive for 41 and 35 cases, respectively. Most (73%) patients were HIV-positive; among those, 51% had a CD4 cell count range of 200-499 cells/mm3. Notably, of the 12 immunocompetent patients, 6 (50%) had a comorbidity, such as diabetes mellitus, alcoholism, drug abuse, psoriasis, or hepatitis.
Studies on malignant syphilis published in 2014–2018.
| No. | References | Race, Sex, Age (years), Sexual orientation | HIV status CD4 count (cells/mm3)/other | Location of the lesions | Onset (before admission) | Serological test results | Histopathology | Immunostaining/microorganism staining | Treatment | JHR | Resolution time/improvement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Dos Santos, et al., 2014 11 | African, male, 27, MSM | HIV+ | Forehead, back, lower limbs | 3 months | VDRL 1:128 | Perivascular mixed inflammatory infiltrate and granuloma in the dermis | NA | Doxycycline 100 mg/12 hours/PO for 3 weeks | − | 3 weeks |
| 340 | Prophylactic hydrocortisone 200 mg IV | ||||||||||
| 2. | Cid, et al., 2014 12 | Male, 42 | HIV+ | Face, scalp, trunk, limbs | 8 days | RPR 1:64 | Abundant histiocytes, giant cells and plasma cells in the dermis | + | Benzathine penicillin G 2.4 mU/week/IM for 3 weeks | − | 3 weeks |
| 442 | IgG + | ||||||||||
| TPPA + | |||||||||||
| 3. | Cid, et al., 2014 12 | Male, 33 | HIV+ | Scalp, cheek, upper limbs, trunk | 5 months | RPR 1:16 | Dense lymphocytes and plasma cells in the dermis | + | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | − | 10 days |
| 1294 | TPPA + | ||||||||||
| 4. | Cid, et al., 2014 12 | Male, 25 | HIV+ | Trunk, scalp | 1 month | RPR 1:64 | Dense lichenoid infiltrate, lymphocytes, histiocytes, and numerous plasma cells on the epidermis | + | Benzathine Penicillin G 2.4 mU/ week/ IM for 3 weeks | − | 2 weeks |
| 210 | TPPA + | ||||||||||
| 5. | Bustos, et al., 2014 13 | Male, 46 | HIV− | Trunk, limbs, genitals, scalp | 2 months | VDRL 1:32, FTA-ABS + | Heavy lymphocytes and histiocytes, abundant plasma cells in the dermis | NA | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | − | 3 weeks |
| Hep B | |||||||||||
| 6. | Requena, et al., 2014 4 | Female, 29 | HIV− | Face, trunk, limbs | 2 weeks | VDRL 1:256 | Dense lymphohistiocytic infiltrate rich in plasma cells, extended into the deep dermis | + | Benzathine penicillin G 2.4 mU/ week /IM for 3 weeks | + | 3 months |
| Psoriasis | Treponemal test + | ||||||||||
| 7. | Kong, et al., 2014 14 | Male, 36, MSM | HIV+ | Face, trunk | 3 weeks | VDRL 1:128 | Granulomatous, lymphocytes, and plasma cells in the dermis | NA | Benzathine penicillin G | − | Improved |
| 8. | Navarrete, et al., 2015 8 | White, male, 25 | HIV+ | Face, trunk, limbs, penis | 3 months | RPR 1:512 | Dense infiltrate of plasma cells, lymphocytes, and histiocytes | + | Benzathine penicillin G 2.4 mU/ week /IM for 3 weeks | − | 1 week |
| 236 | MHA-TP + | ||||||||||
| 9. | Devkota, et al., 2015 15 | African American, male, 20, MSM | HIV+ | Trunk, face, and upper limbs | 1 month | RPR 1:128, | Lichenoid lymphohistiocytic infiltrate with plasma cells | + | Doxycycline 100 mg/ 12 hours/ PO for 3 weeks | + | 3 weeks |
| 276 | FTA-ABS + | ||||||||||
| 10. | Jalili, et al., 2015 16 | White, female, 47 | HIV+ | Upper limbs, lower limbs, trunk, head | 1 month | IgM + | Perivascular and perifollicular infiltration of lymphocytes, many histiocytes and numerous plasma cells in the dermis | − | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | − | 4 weeks |
| 155 | TPPA + | Prophylactic methylprednisolone 40 mg PO 1 hour before penicillin injections | |||||||||
| TPHA 1:1280 | |||||||||||
| VDRL 1:32 | |||||||||||
| 11. | Jiu-Hong Li, et al., 2015 17 | Asian, male, 38, heterosexual | HIV− | Face, trunk, limbs | 1 month | TPPA + | Mixed perivascular infiltrate with neutrophils, lymphocytes, plasma cells, and histiocytes in the dermis | NA | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | − | Improved |
| RPR 1:128 | |||||||||||
| 12. | Jiu-Hong Li, et al., 2015 18 | Asian, male, 35 | HIV− | Face, trunk, limbs | 1 month | TPHA + | Perivascular infiltration of dense neutrophils, lymphocytes, plasma cells and histiocytes | NA | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | − | 4 weeks |
| DM | RPR 1:256 | Prophylactic prednisone 40 mg 1 day before starting penicillin | |||||||||
| 13. | Wei-Ting Chang, et al., 2015 19 | Asian, male, 22 | HIV+ | Face, trunk, limbs | 1 week | RPR 1:4 | Perivascular and interstitial infiltrate of neutrophils, lymphocytes, histiocytes, and some plasma cells in the dermis | NA | Penicillin G aqueous 24 mU/day/ IV for 2 weeks | − | Several days |
| 360 | TPHA 1:320 | ||||||||||
| 14. | Wei-Ting Chang, et al., 2015 19 | Asian, male, 26, MSM | HIV+ | Face, trunk, limbs, genitalia | 3 weeks | RPR 1:512 | Dense lymphohistiocytic infiltrate in upper and middle dermis, numerous plasma cells | + | Penicillin G aqueous 12 mU/day/ IV for 18 days and ciprofloxacin for 7 days | − | 21 days |
| 88 | TPHA 1:20.480 | ||||||||||
| 15. | Hanson, et al., 2015 20 | Male, 45 | HIV+ | Back, groin | 5 weeks | RPR 1:256 | Perivascular granulomatous dermatitis with rare plasma cells | + | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | − | NA |
| 441 | |||||||||||
| 16. | Alves, et al., 2015 21 | Male, 57, MSM | HIV− | Head, neck, trunk, limbs | 4 months | RPR 1:128 | Diffuse dermal inflammatory infiltrate composed of plasma cells, histiocytes, and lymphocytes, forming granulomas in the deeper dermis | NA | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | − | Improved, healed completely in 6 weeks |
| TPHA 1:5120 | |||||||||||
| 17. | Braue, et al., 2015 3 | African-American, male, 36 | HIV+ | Head, face, neck, limbs | 1 month | FTA-ABS + | Prominent dermal infiltrate of epithelioid histiocytes, poorly formed granulomas with giant cells, dermal perifollicular lymphohistiocytic infiltrate | − | Penicillin G aqueous 24 mU/day/ IV for 2 weeks with decreasing dose (because of JHR). then, benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | + | 1 week |
| 450 | RPR 1:1024 | ||||||||||
| 18. | Yamashita, et al., 2015 22 | Asian, male, 40, heterosexual | HIV+ | Trunk, limbs | 4 weeks | RPR + (5.4 RU, card method x4) | Dermal vessels showed venulitis with extravasation of red blood cells, abundant neutrophils, histiocytes, a small number of plasma cells, and eosinophils | + | Penicillin G | − | NA |
| 110 | TP Ag + (2713.6 U) | ||||||||||
| 19. | Martinez, et al., 2016 23 | Male, 54, bisexual | HIV+ | Face, trunk, limbs, scrotum, palms, and soles | 2 weeks | RPR 1:8 | Not mentioned | + | Benzathine penicillin G 2.4 mU/ week/ IM for 3 weeks | + | NA |
| 497 | TPHA 1:2560 | ||||||||||
| 20. | Muylaert, et al., 2016 24 | Female, 50 | HIV− | Face, trunk, limbs | 4 months | VDRL 1:512 | Confirmed secondary syphilis | NA | Benzathine penicillin G 2.4 mU then Ceftriaxone for 14 days | + | 2 weeks |
| alcoholic | FTA-ABS + | ||||||||||
| drug user | IgG + | ||||||||||
| IgM + | |||||||||||
| 21. | Ortigosa, et al., 2016 25 | White, female, 53 | HIV− | Head, chest, limbs | 20 days | VDRL 1:8 | A cluster of non-caseating granulomas, plasma cells, rare eosinophils, endothelial swelling in the dermis | NA | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks and prednisone 60 mg PO daily | − | NA |
| DM | FTA-ABS + | ||||||||||
| 22. | Borges-costa, 2016 26 | White, male, 42, heterosexual | HIV+ | Trunk, upper limbs | 2 weeks | TPHA and VDRL >1:256 | NA | NA | Benzathine penicillin G 2.4 mU/IM | − | Improved |
| 435 | |||||||||||
| 23. | Borges-costa, 2016 26 | White, male, 42, heterosexual | HIV+ | Trunk, upper limbs | 2-8 weeks | TPHA and VDRL >1:256 | NA | NA | Benzathine penicillin G 2.4 mU/IM | − | Improved |
| 435 | |||||||||||
| 24. | Borges-costa, 2016 26 | White, male, 42, heterosexual | HIV+ | Head, trunk, upper limbs | 2 months | TPHA and VDRL >1:256 | NA | NA | Benzathine penicillin G 2.4 mU/IM | − | Improved |
| 435 | |||||||||||
| 25. | Sammet and Draenert, 2016 27 | Male, 40, bisexual | HIV+ | Trunk, generalized | 3 previous episodes | TPPA | NA | NA | Ceftriaxone 2 g/day/ IV for 3 weeks | − | 1 week |
| 360 | 320.000 (2010) | Prophylactic prednisolone 1 mg/kg PO single dose | |||||||||
| 1.28 mill (2012) | |||||||||||
| 320.000 (2014) | |||||||||||
| RPR | |||||||||||
| 26. | Krase, et al., 2016 28 | Asian, male, 43 | HIV− | Trunk, limbs | Several months | FTA-ABS + | Perivascular infiltrate of lymphocytes, numerous plasma cells, and scattered eosinophils | NA | Benzathine penicillin G 2.4 mU/ week /IM for 3 weeks | − | Healed completely in 3 months |
| CKD | RPR 1:4 | ||||||||||
| DM | |||||||||||
| 27. | Delgado and Caceres, 2017 29 | Male, 25 | HIV+ | Face, chest, limbs | 1 month | RPR 1:128, FTA-ABS + | Chronic, granulomatous, noncaseating infiltrate with plasma cells in the dermis | NA | Benzathine penicillin G | − | Improved |
| 108 | |||||||||||
| 28. | Mohan, et al., 2017 30 | Male, 36 | HIV+ | Face, trunk, limbs | 6 weeks | RPR 1:64 | Lichenoid, psoriasiform, granulomatous dermatitis | + | Doxycycline 100 mg bd PO | − | 1 week |
| 57 | |||||||||||
| 29. | Rao, et al., 2017 31 | Asian, male, 35, heterosexual | HIV− | Face, trunk, limbs | 1 month | VDRL 1:32 | A dense collection of neutrophils, lymphocytes, few plasma cells in the dermis, PMN cells in the vessels wall (endarteritis obliterans) | NA | Benzathine penicillin G 2.4 mU/ week IM | − | Improved |
| TPHA 1:60 | |||||||||||
| 30. | Johnson and Spivak, 2017 32 | Male, 41 | HIV+ | Right foot, left chest | 9 months | RPR 1:1024 | Abundance of plasma cells and endothelial hyperplasia | − | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | − | 72 hours |
| 629 | |||||||||||
| 31. | Mena Lora, et al., 2017 33 | Male, 58, MSM | HIV+ | Trunk, limbs, testicles | 2 weeks | RPR 1:256 | Dense dermal plasma cell infiltrates with an overlying purulent serum crust and reactive hyperplastic epidermal changes | − | Benzathine penicillin G 2.4 mU/ week/IM single dose | − | 1 week |
| 463 | |||||||||||
| 32. | Faraone and Fortini, 2017 34 | White, female, 86, Heterosexual | HIV− | Tongue, face, trunk, limbs | 4 months | TPHA 1:10.240 | Epithelial ulceration and an intense perivascular inflammatory infiltrate of the lamina propria, rich in plasma cells | NA | Ceftriaxone for 2 weeks | − | Rapid (healed completely in 1 month) |
| VDRL + | |||||||||||
| 33. | Gevorgyan, et al., 2017 35 | Male, 41 | HIV+ | Face, trunk, limbs, scalp, soles | 4 months | TP Ab + | NA | NA | Benzathine penicillin G 2.4 mU/ week /IM for 3 weeks | + | 2 days (healed completely in 1 month) |
| 101 | RPR 1:64 | ||||||||||
| Drug user | |||||||||||
| 34. | Zanella, et al., 2017 2 | Male, 42 | HIV+ | Face, trunk, limbs, palmoplantar, oral and genital mucosa | 2 months | VDRL 1:128 | Lymphohistiocytic infiltrate with eosinophils and leucocytes, and epithelioid cells with multinucleated giant cells | NA | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | + | Improved after treatment |
| 140 | TPHA + | ||||||||||
| 35. | Zanella, et al., 2017 2 | Female, 42 | HIV+ | Face, tongue, back | 2 months | VDRL 1:64 | Deep perivascular dermatitis | NA | Ceftriaxone 2 g/day for 2 weeks | − | Improved after treatment |
| 586 | TPHA + | Prophylactic corticosteroids | |||||||||
| 36. | Yap, et al., 2018 36 | Male, 71 | HIV+ | Trunk, limb, palms, and soles | 2 weeks | RPR 1:256 | Epidermal ulceration with a dense dermal infiltrate consisting predominantly of mononuclear cells, abundant histiocytes, and plasma cells | NA | Aqueous penicillin G 12 mU/day/ IV for 15 days | − | 2 weeks |
| 252 | TPPA + | Prophylactic prednisolone 60 mg/day for 5 days | |||||||||
| 37. | Sun, et al., 2018 37 | Asian, male, 23 | HIV+ | Face, trunk, limbs | 2 months | Treponemal test + | NA | + | Doxycycline 100 mg/12 hours/ PO for 2 weeks | − | 2 weeks |
| Decreased CD4 | |||||||||||
| 38. | Yang, et al., 2018 38 | Asian, male, 52 | HIV+ | Face | 6 months | Treponemal test + | NA | + | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | − | 1 week |
| 39. | Fustà-Novell X, et al. 2018 39 | Hispanic, male, 39, MSM | HIV+ | Palmoplantar, face, trunk, scalp | 2 weeks | VDRL 1:128 | Dermatitis lichenoid (lymphocytes, histiocytes, plasma cells). | + | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | + | Improved |
| 171 | |||||||||||
| 40. | Fustà-Novell X, et al., 2018 39 | Hispanic, male, 36, MSM | HIV+ | Palmoplantar, nail, scalp, face | 3 weeks | VDRL 1:250 | Dermatitis spongiosa (histiocytes, plasma cells). | + | Penicillin G aqueous 24 mU/day/ IV for 15 days | + | Improved |
| 250 | |||||||||||
| 41. | Fustà-Novell X, et al., 2018 39 | Hispanic, male, 54, MSM | HIV+ | Palmoplantar, limbs | 1 month | VDRL 1:512 | Dermatitis lichenoid (lymphocytes, histiocytes, plasma cells). | − | Benzathine penicillin G 2.4 mU/ week/IM for 3 weeks | − | Improved |
| 697 | |||||||||||
| 42. | Fustà-Novell X, et al., 2018 39 | Hispanic, male, 26, MSM | HIV+ | Palmoplantar, scalp, limbs | 1 month | VDRL 1:256 | Dermatitis abscessed (lymphocytes, histiocytes, plasma cells). | − | Benzathine penicillin G 2.4 mU/ IM single dose | − | Improved |
| 790 | |||||||||||
| 43. | Mitteldorf, et al., 2018 7 | Caucasian, male, 42 | HIV+ | Trunk | 6 weeks | NA | Lichenoid infiltrate, histiocytes. and plasma cells in the dermis | + | Penicillin G aqueous 30 mU/ day/ IV for 2 weeks | − | Few weeks |
| 312 | Prophylactic prednisone 60 mg single dose | ||||||||||
| 44. | Pradhan, et al., 2018 9 | Female, 35, heterosexual | HIV− | Face, trunk, limbs, genital, palm, sole, scalp | 3 weeks | VDRL 1:64 | Plasma cells in the vessel wall and thrombosed vessels in the dermis with endarteritis | NA | Doxycycline 100 mg/12 hours/ PO for 3 weeks | − | 2 weeks |
| TPHA 1:160 | |||||||||||
| 45. | Rockwood, and Nwokolo, 2018 10 | White, male, 41, heterosexual | HIV− | Face, trunk, limbs, left testicular mass | 2 weeks | TPPA + | Chronic epididymo-orchitis with occasional poorly formed granulomas | + | Doxycycline 100 mg/12 hours/ PO for 4 weeks | − | 4 weeks |
| RPR 1:256 |
Abbreviations: FTA-ABS, fluorescent treponemal antibody absorbed; IgG, immunoglobulin G; IgM, immunoglobulin M; JHR, Jarisch-Herxheimer reaction; IM; intramuscular; MSM, men who have sex with men; NA, not available; PO, per os (by mouth); RPR, rapid plasma regain; TP Ab, Treponema pallidum antibody; TPHA, Treponema pallidum hemagglutination test; TPPA, Treponema pallidum particle agglutination; VDRL, Venereal Diseases Research Laboratory.
Skin lesions were reported and verified by histology in 37 of the 45 reviewed cases (Table 2). The most commonly reported histopathology features are lymphohistiocytic dermal infiltrate composed of plasma cells. Data on immunostaining and microorganism staining were given for 23 cases; T. pallidum was detected in 17 of those patients. In the remaining 6 patients, no spirochetes were detected (Table 2).
Treatment and outcomeApproximately two-thirds (64%) of the patients were treated with intramuscular benzathine penicillin G, and the remaining patients were treated with alternatives including as intravenous aqueous penicillin G, oral doxycycline, or intravenous ceftriaxone (Table 1). Nine patients (20%) experienced JHRs, of whom 7 were treated with prophylactic corticosteroids (Table 2).
DiscussionWe reviewed published case reports on 45 patients with MS, published in 2014-2018. It is well recognized that MS is commonly observed in immunosuppressed patients such as those with HIV infection. The pathogenesis of MS is unknown, but it is generally believed that immunosuppression due to HIV coinfection enables T. pallidum to become more virulent. Notably however, most of the HIV-positive patients had a CD cell count >200 cells/mm3, and thus, they were not deeply immunosuppressed.12 The loss of CD4 T cells that occurs as a result of HIV infection or other conditions4 leads to a greater action of cytotoxic T cells and neutrophils on the skin2 in MS. As a result, MS differs from conventional syphilis that occurs in individuals with intact immune systems. This reasoning is consistent with its occurrence in patients with comorbidities or debilitating disease as a cofactor. Our review revealed that 6 of the 12 HIV-negative patients (50%) had comorbidities such as diabetes mellitus, alcoholism, drug abuse, psoriasis, and hepatitis, which could have affected their immune function. The occurrence of MS in HIV-negative patients with comorbidities raises the possibility of aberrant immune response caused by these systemic conditions triggering a more severe skin manifestation and the possibility of a more virulent strains of Treponema, which already considered by other authors.4,32,38,39
MS is often seen in association with high nontreponemal titers and systemic symptoms.5 To guide the clinical diagnosis, in 1969, Fisher et al., proposed 4 criteria in order to identify this rare variant of syphilis, namely: (1) Comparable gross and microscopic morphology; a high titer serologic test for syphilis; a Jarisch-Herxheimer reactions (JHR); and a dramatic response to antibiotic therapy.40
Diagnosis by skin biopsy in affected patients is challenging because spirochetes are generally sparse in the skin lesions. Skin biopsy is, however, a recommended procedure to exclude other bacterial, fungal, and mycobacterial infections. Data obtained from special staining and dark-field microscopy may be insufficient to make a histologic diagnosis,33,40 but microscopic evaluation may reveal nonspecific inflammatory findings, such as histological patterns of dermal infiltrate with plasma cells and lymphocytes, sometimes granulomatous and vascular damage. Our review revealed that lymphohistiocytic dermal infiltrate with plasma cells was the most commonly reported histological feature.12 Immunohistochemistry is superior to silver stains for detecting spirochetes, but it is not always be available as a routine procedure.33
An increase in the incidence of JHR has been described both in patients with MS and in patients with HIV in general. Yang et al describe a rate of incidence of JHR as high as 34.6% in HIV-infected patients.41 In our review, JHR was reported in only 9 patients (20%), and of these 9 patients, 7 were provided with prophylactic corticosteroids. JHR is a transient immunological phenomenon seen commonly in patients during treatment of secondary syphilis; it manifests with constitutional symptoms such as fever, chills, headache, and myalgia, in addition to exacerbation of existing cutaneous lesions. The reaction usually occurs hours after the administration of an appropriate antibiotic and normally resolves without any intervention within 24 hours. JHR is more severe when the number of pathogens is abundant, consistent with a high-titer serological test being part of MS criteria. It should be managed symptomatically and does not require discontinuation of the appropriate antimicrobial treatment. Corticosteroids have been used to prevent the reaction, but there is no conclusive evidence regarding their benefit.35,42
Currently, there is no specific recommendations for MS treatment. The most commonly used treatment regimen is the same as that used for late latent syphilis (3 consecutive weekly intramuscular injections of benzathine penicillin, 2.4 million units/dose). In case of allergy to penicillin, treatment with ceftriaxone can be used. In resistant cases or relapses, prolonged therapy with high doses of penicillin is suggested.12,25 About 80% of the patients in our review were treated with penicillin, either intramuscularly or intravenously as aqueous solution, and all the patients had a rapid improvement in their condition following antibiotic treatment.
ConclusionOur review included 45 case reports of MS published from 2014-2018, available on the PubMed and/or Google Scholar databases. Of the patients, 74% were HIV positive. Of the HIV-negative patients, half had a comorbidity such as diabetes mellitus, alcoholism, drug abuse, psoriasis, hepatitis. The majority of cases occurred in men (84%), the median age of presentation is 41 years with the 40-44 years old age group being the most frequently affected. The most frequent cutaneous manifestations were ulceronodular lesions with an adherent crust on the central surface affecting the face, trunk, and limbs. with half of patients with HIV infection having a CD4 cell count in the 200-499 cells/mm3 range. The majority of patients were treated with benzathine penicillin G or aqueous penicillin G, and all patients experienced a rapid clinical improvement following antibiotic therapy. JHR was reported in 20% of patients, despite the majority of them having been given prophylactic corticosteroids. In the face of the increased number of cases of this rare form of syphilis regardless of the immune status, dermatologists and general practitioners should be attentive to the occurrence of MS in order to allow early diagnosis and treatment, hence reducing their morbidity.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Hindawi Editage (http://hindawi.editage.com/) for English language editing.
Please cite this article as: Wibisono O, Idrus I, Djawad K. Sífilis maligna: revisión sistemática de los casos publicados entre los años 2014-2018. Actas Dermosifiliogr. 2021. https://doi.org/10.1016/j.ad.2021.02.011