In the oncologic management of hormone-dependent tumors, drugs with pronounced antihormonal effects are frequently used. Examples include prostate cancer and breast cancer, in which various antiandrogenic agents are administered.
Patients receiving treatment with antiandrogenic effects may develop androgenetic alopecia (AGA) due to the therapy-induced hormonal state. In hormone-dependent neoplasms of the male genital system, such as prostate cancer, the expected alopecia pattern is that of typical male AGA, predominantly involving the frontotemporal regions. Conversely, in women with breast cancer undergoing antiandrogenic therapy, the typical pattern of female AGA – generally with thinning over the vertex – is anticipated. However, it is not uncommon for these patients to develop AGA with a characteristically male distribution pattern, particularly affecting the frontotemporal areas.
We recently observed such a case in a 45-year-old woman who presented with progressive hair loss in the frontal scalp over the previous two years, with more marked worsening during the last year. The patient denied traction hairstyles or practices involving hair pulling. Her past medical history included breast cancer six years earlier, treated with 12 cycles of paclitaxel, followed by 4 cycles of doxorubicin and cyclophosphamide, and subsequent radiotherapy. This oncologic treatment had concluded six years prior. She later began hormonal therapy consisting of goserelin (Zoladex®).
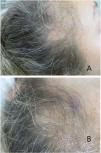
Physical examination revealed marked reduction in hair density in both temporal regions, along with bitemporal recession of the frontal hairline (Fig. 1A and B). The hair-pull test was negative, and no nail abnormalities were observed. Trichoscopy demonstrated marked anisotrichosis with only one hair shaft per follicular unit in the affected areas. No perifollicular hyperkeratosis, black dots, broken hairs, or peripilar cylinders were identified.
Although alopecia was considered highly likely to be due to hormonal therapy, a biopsy was performed due to a slightly indurated sensation on palpation of the alopecic areas to exclude an inflammatory or scarring alopecia.
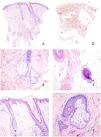
Histologic examination showed a reduction in follicular density with follicular miniaturization (Fig. 2A). No sebaceous gland atrophy was observed (Fig. 2B). A minimal superficial perivascular lymphohistiocytic infiltrate was noted, without evidence of dermo-follicular or dermo-epidermal interface damage. There was no peri-isthmic fibrosis (Fig. 2C), and the elastic fiber network was preserved (Fig. 2D). No inflammatory infiltrates were seen either in the inferior portion of the follicles or in the hair bulbs (Fig. 2E). Some follicles showed clear miniaturization of hair shafts (Fig. 2F). PAS staining did not reveal fungal elements, nor were mucin deposits identified in the reticular dermis.
(A) Panoramic view of the biopsy showing follicular miniaturization with a minimal superficial perivascular inflammatory infiltrate and no evidence of scarring. (B) Preserved sebaceous glands without signs of atrophy or destruction. (C) Detailed view of the follicular isthmus demonstrating preservation without interface damage or peri-isthmic fibrosis. The follicle on the left shows marked miniaturization. (D) Orcein histochemical staining for elastic fibers, demonstrating preservation of the reticular network without signs of destruction or scarring. (E) No abnormalities were observed in the follicular bulbs; notably, no peribulbar lymphocytic infiltrates were identified. (F) At sebaceous duct opening level inside a pilosebaceous unit, a significant reduction in hair shaft diameter is evident in a miniaturized follicle.
Based on these findings, along with the clinical context, the diagnosis of a non-inflammatory, non-scarring alopecia with follicular miniaturization – consistent with therapy-induced AGA – was established.
The presence of a male-pattern distribution of alopecia in a woman in response to antiandrogenic therapy supports a recently proposed interpretation of divergent pathogenic pathways in male and female AGA.1 According to this model, the predominant pathogenic mechanism in men involves follicular miniaturization, whereas in women it consists of a reduction in the number of hairs per follicular unit. This may explain why drugs that induce follicular miniaturization predominantly produce a male-pattern alopecia rather than the typical female pattern, in both men and women.
This patient had been treated with goserelin and tamoxifen. While tamoxifen is an estrogen-receptor antagonist, goserelin initially stimulates gonadotropin-releasing hormone receptors, causing a transient increase in luteinizing hormone (LH) and follicle-stimulating hormone (FSH), before ultimately desensitizing pituitary receptors with continued use, leading to sustained suppression of LH and FSH and decreased testosterone and estrogen levels.2,3 These two agents may induce alopecia,4,5 although usually less severe than that caused by chemotherapeutic agents and generally under-recognized.4
We found only a single published case with histopathologic description of alopecia in a patient treated with goserelin for prostate cancer. Interestingly, that case demonstrated features of scarring alopecia consistent with frontal fibrosing alopecia.6
This case, however, carries a limitation in attributing the alopecia solely to hormonal therapy: the patient had previously been treated with paclitaxel, a taxane. This class of drugs – particularly docetaxel – has been associated with androgenetic-type alopecia in both male and female patterns, even years after treatment.7,8