Psoriasis is a chronic immune inflammatory disease that affects 1–3% of the global population. Although it is characterized by the presence of well-demarcated erythematous and scaly plaques, preferentially located on the elbows, knees, and scalp, it can also affect the palms, soles, nails, and joints1. Currently, there is a wide variety of biologic therapies targeting different molecular pathways with the aim of specifically addressing the mechanisms involved in the pathophysiology of the disease. Persistence is defined as the time from the first to the last administered dose before definitive treatment discontinuation.1 The purpose of this real-world study is to analyze the persistence, safety and efficacy profile of guselkumab in psoriasis in the routine daily practice with longer follow-up. We conducted a retrospective observational study from April 2019 to September 2023 at 2 hospitals in the Valencia Region (Spain). Inclusion criteria were adult patients with moderate-to-severe psoriasis who completed the guselkumab label induction and maintenance dosage. Patients with psoriatic arthritis were excluded. Patients were categorized as biologic-naïve or biologic-experienced. The following variables were collected: sex, age, diagnosis, previous biological treatments, start date of guselkumab, dosage regimen, psoriasis area severity index (PASI), adverse events and reason for discontinuation. Dermatology life quality index (DLQI) was used to evaluate the patients’ health-related quality of life. Persistence on guselkumab was calculated based on the dates of initiation and end of treatment. If the patient was still on therapy, persistence was calculated based on the date when the follow-up ended (September 2023).2 Data were obtained from the Pharmacy Department and the patients’ electronic health record. Persistence for biologic therapy was calculated using Kaplan–Meier estimates. Adherence was measured using the medication possession ratio (MPR) from the dispensing records of the hospital pharmacy department.3 The study was approved by the hospital Clinical Research Ethics Committee, and conducted in full compliance with the principles set forth in the Declaration of Helsinki.
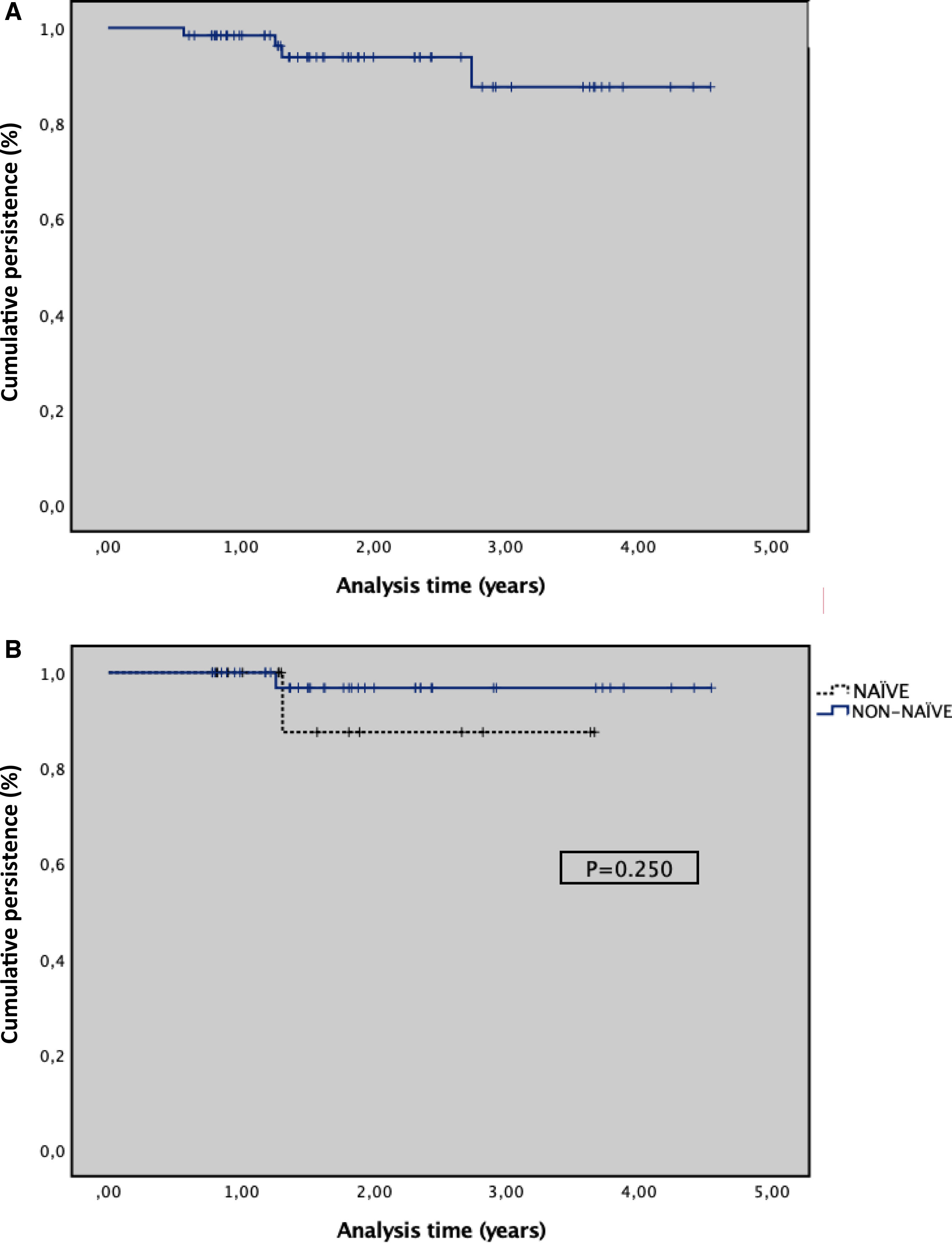
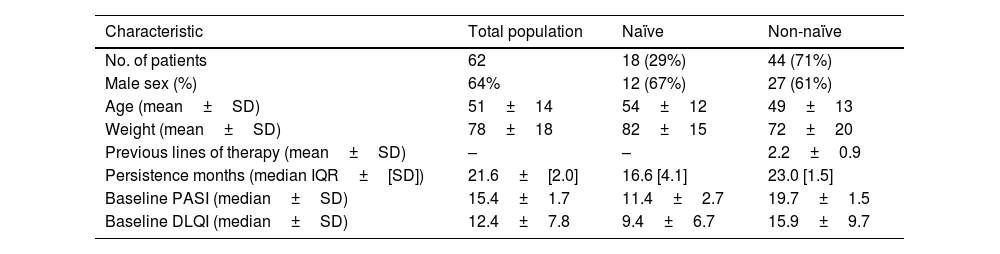
A total of 62 adult patients with moderate-to-severe psoriasis who received guselkumab were included (Table 1). Eighteen (29.0%) patients were naïve to biologic therapy, 26 (42.0%) patients received 1 biologic agent, and 18 (29.0%) at least 2. Baseline PASI score was 15.4±1.7 and patients received a mean of 1.9±0.9 previous biologic agents. The median disease duration was 7.9±0.9 years, with patients presenting a mean of 1.9±0.9 comorbidities. The mean duration of family history of psoriasis was 5.9±2.7 years, and 45% of patients reported a family history of psoriasis. The cumulative persistence with treatment for the overall population was 93.5% (95%CI, 87.3–99.8%), with a median follow-up time of 20.4 months (7.3–54.5). The 1-year probability of persistence, calculated using the Kaplan–Meier method, was 98.4% (95% CI, 95.2–99.9%); at 2 years, 95.6% (95% CI, 86.6–100%); and at 3 years, 91.8%. The median (IQR) duration of persistence with guselkumab treatment was 21.6 (2.0) months, ranging from 17.6 to 25.5 months. In the subgroup analysis, biologic-experienced (non-naïve) patients demonstrated a persistence of 23.0±[1.5] months vs 16.6±[4.1] months for biologic-naïve patients. However, this difference was not statistically significant (p=.250) (Fig. 1). A total of 62.2% of patients achieved a PASI 100, followed by 81.3% with a PASI 90, and 93.6% with a PASI 75. The median DLQI score decreased from 12.4±7.8 at baseline to 3.5±2.7 after 12 months of treatment. Five (8.1%) patients discontinued guselkumab treatment for the following reasons: 3 (4.8%) due to a lack of effectiveness, 1 due to elevated transaminase levels, and 1 (1.6%) due to pregnancy.
Demographic and disease characteristics at baseline.
| Characteristic | Total population | Naïve | Non-naïve |
|---|---|---|---|
| No. of patients | 62 | 18 (29%) | 44 (71%) |
| Male sex (%) | 64% | 12 (67%) | 27 (61%) |
| Age (mean±SD) | 51±14 | 54±12 | 49±13 |
| Weight (mean±SD) | 78±18 | 82±15 | 72±20 |
| Previous lines of therapy (mean±SD) | – | – | 2.2±0.9 |
| Persistence months (median IQR±[SD]) | 21.6±[2.0] | 16.6 [4.1] | 23.0 [1.5] |
| Baseline PASI (median±SD) | 15.4±1.7 | 11.4±2.7 | 19.7±1.5 |
| Baseline DLQI (median±SD) | 12.4±7.8 | 9.4±6.7 | 15.9±9.7 |
SD: standard deviation; IQR: inter-quartile range.
At the end of the study, a total of 57 (91.9%) patients were still on therapy, 10 (16.1%) patients were on a dosage of 100mg every 10 weeks and 5 (8.1%) on a dosage of 100mg every 12 weeks. Optimization criteria included patients treated with guselkumab who achieved and maintained clinical remission for 6 to 12 months. The mean adherence rate was 90.4±6.4%.
Persistence can be considered a surrogate for treatment success, dependent on safety and efficacy, comfort of administration, and patient satisfaction.4 Our study has shown a high persistence with guselkumab at 1, 2 and 3 years. We report 3-year persistence rates exceeding 90%, supporting the safety, efficacy, and patient satisfaction associated with guselkumab in routine clinical practice for the treatment of moderate-to-severe psoriasis. Comparable persistence rates for guselkumab were observed in a multicenter 2-year drug survival study.5 PASI 100 was achieved in 62% of patients vs 34% in the VOYAGE 26 clinical trials, respectively. These differences may be due to the fact that the patients included in our study had a much lower median baseline PASI vs the patients from the pivotal trials. Similar to former studies, 24.1% of patients received a reduced dose of guselkumab.8 Guselkumab had a manageable adverse event rate and only 3.2% of patients had to discontinue treatment for safety reasons, similar to other studies.7,9 The difference in persistence between naïve and non-naïve patients was not statistically significant (p=0.250), the slightly higher persistence observed in the non-naïve group may suggest greater motivation or familiarity with biologic treatments. Prior experience could facilitate better adherence through more realistic expectations and improved disease self-management. While inconclusive, this finding warrants further investigation in larger cohorts considering psychosocial factors. These differences compared with the pivotal trials5,6 may be due to the small sample size, and immaturity of the data in the naïve group, as these were patients who started treatment later. A strength of this study is that patients were considered adherent to guselkumab based on the MPR data, which makes the persistence findings of the study more generalizable and reliable for real-world clinical practice.10 Limitations of this study include the limited sample size and its observational, retrospective design. Additionally, the absence of a control group, potential variability in clinical practice across participant hospitals, and the possible influence of treatment optimization strategies (eg, extending dosing intervals to 10–12 weeks) on persistence outcomes should be acknowledged as factors that may affect the generalizability and interpretation of the results. In conclusion, guselkumab demonstrated high persistence up to 3 years, suggesting a good safety and efficacy profile in real-world practice.
Conflict of interestThe authors declare that they have no conflict of interest.