The 4-item Psoriatic Arthritis Uncluttered Screening Evaluation (PURE-4) questionnaire is a useful tool for identifying patients with suspected psoriatic arthritis before referring them to a rheumatology department for confirmation. The original English version has good discriminant validity (sensitivity, 85.7%; specificity 83.6%). We aimed to produce an adapted Spanish version of the PURE-4 for validation and use in Spain.
Material and methodWe applied the method recommended by the International Society for Pharmacoeconomic and Outcome Research for the cultural adaptation of patient-centered measurement tools. The phases in the processes involved forward translation, reconciliation, back translation review, harmonization, cognitive debriefing and review, and proofreading.
ResultsWe obtained the permission of the author of the original questionnaire. Two native-speaking translators translated the questionnaire into Spanish. Small changes, mainly in the way the items were expressed, were then made in order to reconcile the 2 translations. The questionnaire was then back translated to English and revised to achieve a version equivalent to the original. A Spanish translation derived from the revision was tested for understandability in 7 patients, and the final Spanish version was then produced. During the translation phases, the project manager and a scientific committee made up of a dermatologist and a rheumatologist reviewed the different versions. Team members exchanged information throughout the process, providing for harmonization and the quality control that guaranteed conceptual equivalence.
ConclusionsThis adaptation of the PURE-4 questionnaire for use in Spain has been the first step toward using it in routine clinical practice. The standardized method we used ensures that the Spanish and the original versions are equivalent.
El cuestionario PURE-4 puede considerarse una herramienta útil para identificar pacientes con posible artritis psoriásica y derivarlos al servicio de reumatología para confirmar el diagnóstico. La versión original en inglés presenta alta validez discriminatoria (85,7% sensibilidad, 83,6% especificidad). El objetivo de este trabajo es adaptarlo para población española como paso previo a su validación.
Material y métodoSe aplicó la metodología recomendada por la International Society Pharmacoeconomic and Outcome Research (ISPOR) para adaptaciones culturales de medidas centradas en el paciente. Fases: preparación, traducción, reconciliación, retrotraducción/revisión, armonización, test de comprensión/revisión, corrección de pruebas.
ResultadosEn la preparación se obtuvo el permiso del autor del cuestionario original. Dos traductores nativos realizaron la traducción del cuestionario original al español. En la reconciliación se realizaron pequeñas modificaciones, principalmente en el enunciado de los ítems. Se realizó retrotraducción al inglés, logrando una versión equivalente al cuestionario original. La versión española derivada se administró en el test de comprensión a 7 pacientes, obteniéndose la versión final en español. Durante las traducciones, el responsable del proyecto y un comité científico formado por un dermatólogo y un reumatólogo revisaron las diferentes versiones. Los intercambios de información entre el equipo durante todo el proceso integraron la fase de armonización, siendo un control de calidad continuo que garantizó la equivalencia conceptual de las traducciones.
ConclusionesLa adaptación del cuestionario PURE-4 para población española constituye la primera etapa para su uso en práctica clínica habitual. La metodología estandarizada garantiza la equivalencia entre la versión española y la original.
Psoriatic arthritis (PsA) is an inflammatory arthritis often associated with psoriasis.1 The clinical course is variable, ranging from slow progression to one that is rapid and destructive. Delay in diagnosis and treatment can lead to irreversible erosive arthropathy, causing physical disability and bone deformity.2
In Spain, the estimated prevalence of psoriasis is 2.3%,3 and it is calculated that 20% to 30% of patients with the disease may also present PsA.4 Furthermore, some studies suggest that severe forms of psoriasis are associated with a higher risk of developing PsA.5 However, a high prevalence of undiagnosed PsA (15%-40%) has been reported among patients with psoriasis.1,6 One of the factors that could explain underdiagnosis of PsA1,6 is the difficulty dermatologists face to differentiate between PsA and other forms of arthritis in those patients with psoriasis who complain of joint pain.7,8
The high rate of underdiagnosis points to a need to improve the detection, assessment, diagnosis, and treatment of PsA. To achieve this, better screening for PsA in patients with psoriasis is necessary, as appropriate and timely care increases the chances of controlling the negative impact on health-related quality of life.9,10
To enable early detection of PsA, screening tools are needed to identify patients at risk of developing the disease, particarly in primary care and dermatology clinics, given that skin manifestations occur prior to PsA in 84% of cases.3 This requires sensitive tools that can be readily applied in clinical practice to identify the first signs and symptoms of PsA.
The tools currently available for screening patients for PsA (Psoriasis Epidemiology Screening Tool [PEST],11 Toronto Psoriatic Arthritis Screen [ToPAS],12 Psoriatic Arthritis Screening Evaluation [PASE],13,14 and Early Arthritis for Psoriatic Patients [EARP] questionnaire15) have a sensitivity and specificity greater than 85%.16 Of all the tools mentioned, the EARP questionnaire is the one that has been shown to be the most sensitive in comparison with ToPAS, PEST, and PASE.16,17 However, all these tools have limitations when implemented in dermatology clinics, given their complexity and/or the time needed to correctly record the different items included.18 A meta-analysis performed by Iragorri et al.16 showed that additional data would be needed to determine the extent to which these tools could be efficient for screening purposes.
Recently, a new scale has been published for detection of PsA: the PURE-4 (4-item Psoriatic arthritis UnclutteRed screening Evaluation) questionnaire.18 This questionnaire only includes 4 items. It has been developed as a brief and simple tool to screen for patients with symptoms indicative of PsA, for whom further evaluation by a rheumatologist would be justified.18
The PURE-4 questionnaire18 was developed based on a literature review of symptoms associated with PsA. Subsequently, a group of dermatologists and rheumatologists agreed by consensus on 23 items that would cover the characteristics of PsA, such as presence of psoriasis, pain in different areas of the body (peripheral, axial, buttocks, chest wall, fingers and toes, heels) and signs of inflammation that occur in PsA (morning stiffness, swollen and/or sensitive joints). The 23 items were administered to a sample of 137 patients diagnosed with psoriasis (patients with confirmed diagnosis of PsA were excluded). A logistic regression model detected the 4 items that best differentiated between patients with and without suspicion of PsA. The 4 items were evocative signs of dactylitis, inflammatory heal pain, bilateral buttock pain, and peripheral joint pain with swelling before 50 years of age. These 4 times require a yes or no answer and a final score is calculated based on the number of positive responses (total score ranging from 0 to 4). The questionnaire showed good discriminatory power (area under curve of 87.6%). In the threshold of ≥ 1 positive response, the PURE-4 questionnaire achieved an excellent sensitivity (85.7%) and specificity (83.6%), with dactylitis being the most specific element (100.0%).18
Currently, this scale has not been validated in the Spanish population. The first step towards such a validation is linguistic adaptation to Spanish as spoken in Spain, in accordance with good translation practices and cultural adaptation to patient reported outcomes (PROs).19
Direct translation of a questionnaire can be associated with errors of interpretation due to cultural differences between countries. Therefore, before application in a population with a different culture and/or language to the original version, it is essential to undertake an appropriate process of cultural adaptation and subsequent validation. For this objective, the guidelines published by the International Society of Pharmacoeconomic and Outcomes Research (ISPOR) establish the framework for translation and cultural adaptation of PRO and standardize its methodology.19 The present article describes the process followed for cultural adaptation of the PURE-4 questionnaire for the Spanish population.
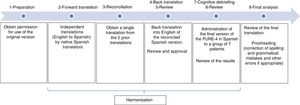
MethodsCultural adaptation of the PURE-4 Questionnaire was performed according to the ISPOR guidelines,19 with the following phases: 1) preparation, 2) forward translation, 3) reconciliation, 4 and 5) back translation and review, 6) harmonization, 7 and 8) cognitive debriefing and review, and 9) proofreading (Fig. 1).
In this phase, the permission of the author of the original questionnaire is requested for the cultural adaptation, inviting them to participate as a consultant during the process. In this case, the author declined to participate.
A working group was established comprising a translator whose native tongue was the original language (English), 2 native Spanish translators, 2 clinical consultants (a dermatologist and a rheumatologist), and a project manager (PM).
The PM presented the conceptual framework of the questionnaire to the team to avoid ambiguity and assist the translators in their work. The PM also presented the distribution of items, the instructions, and the options for response, with the aim of facilitating the conceptual equivalence of the translations.
Phase 2: Forward TranslationTwo professional native Spanish translators who were fluent in English translated the questionnaire literally and independently.
Phase 3: ReconciliationThe PM, with the help of the 2 clinical consultants, determined, for each item, the translation that most exactly captured the original meaning, in a simple and colloquial language, to thus obtain a first version in Spanish of the questionnaire.
Phase 4 and 5: Back Translation and ReviewThe professional native English translator literally translated the reconciled questionnaire to the original language. The PM compared both versions and discussed with the translators the differences identified to detect possible issues with comprehension.
Phase 6: HarmonizationThe harmonization phase comprised information exchange during the entire process, ensuring constant agreement between versions. Instead of considering harmonization an isolated step, this process was included as a continuous quality control to guarantee conceptual equivalence of the translations.
Phase 7 and 8: Cognitive debriefing and reviewIn cognitive debriefing and review, the Spanish version of the questionnaire resulting from the previous phases was administered to 7 patients with psoriasis and no prior diagnosis of PsA who belonged to the Accion Psoriasis (Psoriasis Action) patient group. The mother tongue of these patients was Spanish.
This number of patients is in line with that proposed by the IPSOR guidelines (5–8 participants),19 and is similar to other cultural adaptations.20,21 This number was therefore considered sufficient to determine the level of comprehension of the items and assess possible confounding factors.
The results of cognitive debriefing were reviewed and commented by the working group, leading to a second and final version of the questionnaire in Spanish.
At all times, ethical requirements were met and confidentiality observed. Informed consent was obtained from the patients.
Phase 9: ProofreadingIn this phase, errors that had not been detected earlier were corrected, and spelling and grammar were revised before the instrument was considered apt for use in the target population.
ResultsTable 1 shows all versions of the questionnaire.
Modifications Made to the Items of the PURE-4 Questionnaire.
| Original Version | First Intermediate Version | Second Intermediate Version | Post-Cognitive Debriefing Version | Experts Final Version |
|---|---|---|---|---|
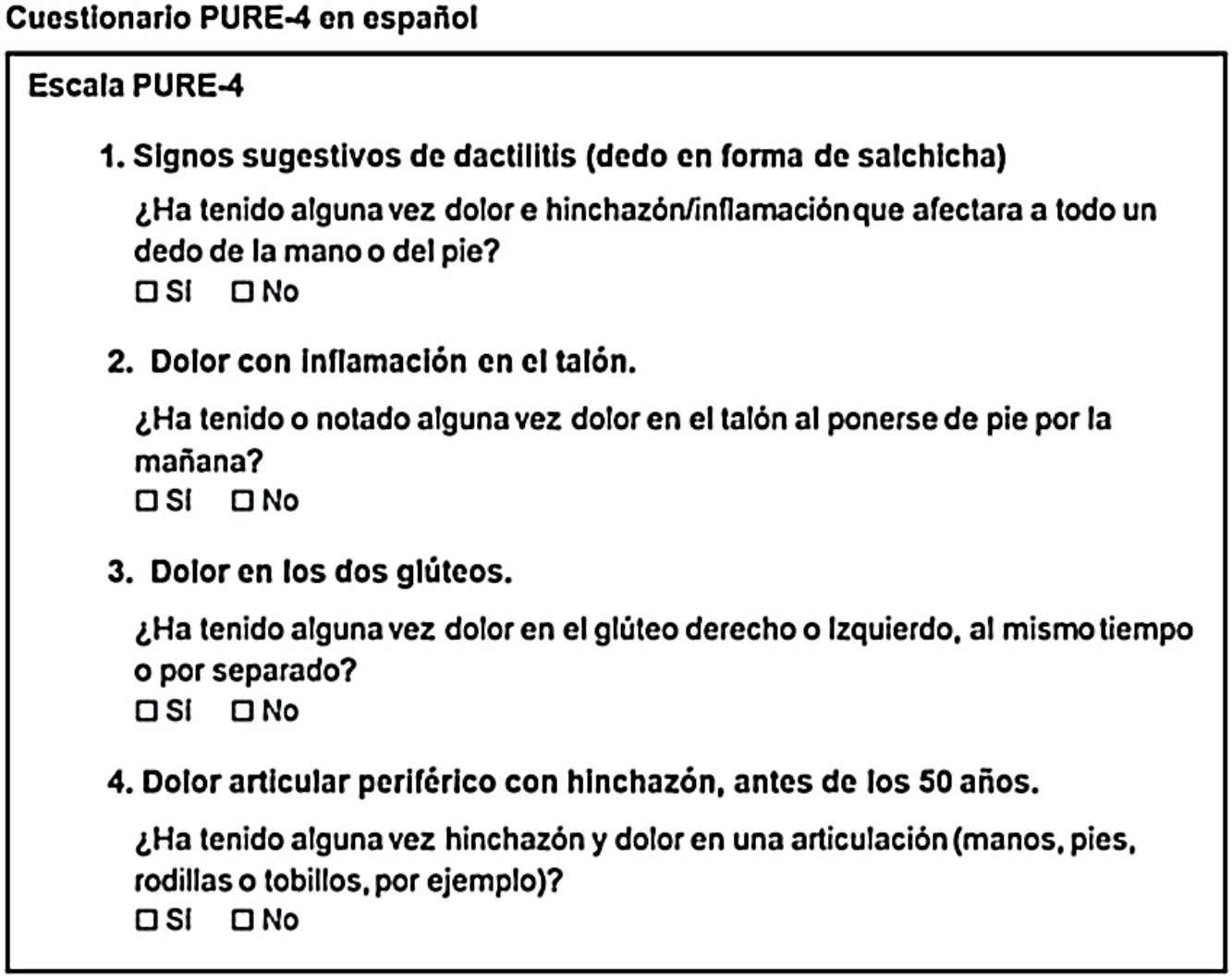
| 1. Evocative signs of dactylitis | 1. Signos evocadores de dactilitis | 1. Signos sugestivos de dactilitis | 1. Signos sugestivos de dactilitis (dedo en forma de salchicha) | 1. Signos sugestivos de dactilitis (dedo en forma de salchicha) |
| Have you ever had a globally swollen and painful finger or toe? | ¿Ha tenido alguna vez dolor o hinchazón general en un dedo de la mano o del pie? | ¿Ha tenido alguna vez dolor e hinchazón que afectara a todo un dedo de la mano o del pie? | ¿Ha tenido alguna vez dolor e hinchazón/inflamación que afectara a todo un dedo de la mano o del pie? | ¿Ha tenido alguna vez dolor e hinchazón/inflamación que afectara a todo un dedo de la mano o del pie? |
| □ Yes □ No | □Sí □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No |
| 2. Inflammatory heel pain | 2. Dolor inflamatorio en el talón | 2. Dolor inflamatorio en el talón | 2. Dolor con inflamación en el talón | 2. Dolor con inflamación en el talón |
| Have you ever had heel pain as soon as you stand up in the morning? | ¿Ha tenido alguna vez dolor en el talón en cuanto se pone de pie por la mañana? | ¿Ha tenido o notado alguna vez dolor en el talón al ponerse de pie por la mañana? | ¿Ha tenido o notado alguna vez dolor e inflamación en el talón al ponerse de pie por la mañana? | ¿Ha tenido o notado alguna vez dolor en el talón al ponerse de pie por la mañana? |
| □ Yes □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No |
| 3. Bilateral buttock pain | 3. Dolor bilateral en los glúteos | 3. Dolor bilateral en los glúteos | 3. Dolor en los dos glúteos | 3. Dolor en los dos glúteos |
| Have you ever had left and right buttock pain, at the same time or not? | ¿Ha tenido alguna vez dolor en el glúteo derecho e izquierdo, al mismo tiempo o por separado? | ¿Ha tenido alguna vez dolor en el glúteo derecho e izquierdo, al mismo tiempo o por separado? | ¿Ha tenido alguna vez dolor en el glúteo derecho o izquierdo, al mismo tiempo o por separado? | ¿Ha tenido alguna vez dolor en el glúteo derecho o izquierdo, al mismo tiempo o por separado? |
| □ Yes □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No |
| 4. Peripheral joint pain with swelling, before 50 year | 4. Dolor articular periférico con hinchazón, antes de los 50 años | 4. Dolor articular periférico con hinchazón, antes de los 50 años | 4. Dolor articular con hinchazón, antes de los 50 años | 4. Dolor articular periférico con hinchazón, antes de los 50 años |
| Have you ever had a globally swollen and painful finger or toe? | ¿Ha tenido alguna vez hinchazón o dolor en una articulación? | ¿Ha tenido alguna vez hinchazón o dolor en una articulación? | ¿Ha tenido alguna vez hinchazón o dolor en una articulación? | ¿Ha tenido alguna vez hinchazón y dolor en una articulación (manos, pies, rodillas o tobillos, por ejemplo)? |
| □ Yes □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No | □ Sí □ No |
Abbreviation: PURE-4, 4-item Psoriatic arthritis UnclutteRed screening Evaluation.
The translators made several comments on the reconciled translations:
- •
Item 3: the end of the original question stated “at the same time or not.” Given that question is talking about bilateral buttock pain the translators interpreted this as meaning “pain in both buttocks at the same time or separately,” although it could also mean “pain in both buttocks at the same time or not” (no pain), but the yes/no responses for this latter option would not be meaningful. Therefore, it was decided to translate this as al mismo tiempo o por separado (at the same time or separately), for which a yes/no answer could be given.
Review of the reconciled translation by the experts gave rise to the first intermediate version of the PURE-4 questionnaire in Spanish.
Back Translation and ReviewThe first intermediate version of the questionnaire in Spanish was back translated to English by a native English translator. The comments of the PM and translators after back translation were:
- •
Item 1: replace the word evocador (evocative) with sugestivo (suggestive).
- •
Item 1: replace hinchazón general en un dedo (globally swollen finger) with hinchazón que afectara a todo un dedo (swelling of an entire finger).
- •
Item 2: notado (noted) should be added, so the back translation would be “had or noted”
- •
Item 2: en cuanto se pone en pie (as soon as you stand up) should be replaced with al ponerse de pie (on standing up).
With these changes, a second intermediate version of the questionnaire in Spanish was obtained.
Cognitive DebriefingThis step is essential to ensure a good level of comprehension of the questionnaire by the target population and to identify possible terms leading to confusion.
A total of 8 patients were recruited, of whom 7 were valid for assessing their comprehension of the PURE-4 questionnaire. Patients were aged between 40 and 74 years, and there were more women than men (57.1%) (Table 2).
Sociodemographic Characteristics of Patients Interviewed and Score Obtained on the PURE-4 Scale.
| Characteristics | Patients (n = 7) |
|---|---|
| Age, Mean (SD) | 60.3 (11.5) |
| Sex, n (%) | |
| Female | 4 (57.1) |
| Male | 3 (42.9) |
| Educational Attainment, n (%) | |
| Higher education | 4 (57.1) |
| High school | 2 (28.6) |
| Elementary school | 1 (14.3) |
| Working Status, n (%) | |
| Retired | 4 (57.1) |
| Working | 3 (42.9) |
| Years Since Diagnosis of Psoriasis, n (%) | |
| > 20 years | 6 (85.7) |
| 15 years | 1 (14.3) |
| Comorbidities | |
| Arthrosisa n (%) | 3 (42.9) |
| PURE-4 score (Scale 0−4), mean (DE) | 1.1 (1.1) |
Abbreviation: PURE-4, 4-item Psoriatic arthritis UnclutteRed screening Evaluation.
The minimum and maximum scores on the PURE-4 scale ranged from 0 to 3 points, with a mean (SD) of 1.1 (1.1). All patients responded to all items, with no answers omitted.
Comments and suggestions were collected from the patients: 32 incidents were identified in total (Table 3).
Comments and Suggestions by the Patients During Cognitive Debriefing.
| Item | Original English Version | Second Intermediate Version (Back Translation) | Patient Comments/Suggestions (Verbatim Expressions by the Patients) | Assessment of the Proposed Changes |
|---|---|---|---|---|
| Title | PURE-4 | PURE-4 Scale | P3: “I don’t know what it is. I would add something about inflammation to the title.” | In general, the patients comment that they do not understand the title and it is meaningless to them. However, there are no suggestions. |
| P5: “This suggests to me a scale that serves for different items. But the title doesn’t mean anything to me.” | It is decided not to change the title and keep the original title. | |||
| P6: “I don’t understand it, I suggest to call it ‘escala de probabilidad de tener artritis psoriásica’ (risk scale for psoriatic arthritis).” | ||||
| P8: “I don’t understand the title, but the name is OK. If you don’t know what it is, you get more response.” (This last point is referring to the fact that if the title does not indicate what the questionnaire is about, this could be something positive as it would encourage the patients to respond out of curiosity.) | ||||
| I1-Title | Evocative signs of dactylitis | Signos sugestivos de dactilitis | P3: “You don’t understand the word dactilitis (dactylitis) if you don’t read the question below.” | In general, the patients have difficulties understanding the term dactilitis (dactylitis). However, their doubts are resolved when they read the question that follows. As an alternative, they suggest putting in parenthesis “sausage finger” so that the patient understands the term better. |
| P4: “I don’t know what dactylitis is. You can get an idea from the question that follows.” | ||||
| P5: “I don’t understand the word dactilitis (dactylitis). You can work it out from the question.” | ||||
| P6: “I would change dactilitis (dactylitis) for another expression that implies swelling or arthrosis of the fingers.” | ||||
| P7: “I would put dactylitis and in parentheses the part about signs suggestive of inflammation or pain.” | ||||
| P8: “I have no idea what dactylitis is. I would change dactylitis for a more colloquial word.” | ||||
| II-Question | Have you ever had a globally swollen and painful finger or toe? | ¿Ha tenido alguna vez dolor e hinchazón que afectara a todo un dedo de la mano o el pie? | P3: “Change hinchazón (swollen) for inflamación (inflammation).” | Most of the patients’ comments suggest using inflamación (inflammation) instead of hinchazón (swollen) or adding both words. It is proposed to include both words in the question. |
| P5: “Inflamación (inflammation) instead of hinchazón (swollen), although the concept of swelling is more colloquial.” | ||||
| P6: “I understand that if I haven’t experienced pain or swelling in the entire figure (‘todo un dedo’), this doesn’t count. I would change reference to the entire finger for a small part of the finger.” | ||||
| P7: “I would perhaps also put inflammation. It would thus refer to swelling or inflammation.” | ||||
| I2-Title | Inflammatory heel pain | Dolor inflamatorio en el talón | P3: “This surprised me because I didn’t think the heel could become inflamed. I associate this with pain. Perhaps I would put ‘Pain caused by inflammation in the heel’.” | Patients are confused by pain and inflammation. Consider whether it is possible to add dolor con inflamación “pain with inflammation.” |
| P5: “I am not clear how you would perceive inflammation. You don’t feel inflammation until it is evident. There is a risk that the patient has felt pain, but not inflammation and does not respond appropriately.” | ||||
| P7:” Pain or discomfort in the heel. But you don’t know whether it is inflammation or just pain (in the heel, it is difficult to know if the heel is inflamed).” | ||||
| I2-Question | Have you ever had heel pain as soon as you stand up in the morning? | ¿Ha tenido o notado alguna vez dolor en el talón al ponerse de pie por la mañana? | P4: “You could add the word inflamación (inflammation): Have you ever had heel pain or inflammation as soon as you stand up in the morning?” | Although only one person suggests adding the word inflamación (inflammation), this could be something to consider, as the word inflammation appears in the title of Question 2 but does not appear in the question. |
| P7: “I understand the question but it should specify whether it refers to acute pain or pain prolonged over time.” | ||||
| I3-Title | Bilateral buttock pain | Dolor bilateral en los glúteos | P1: “I would change buttocks for upper part of the leg. The word buttocks can be confusing.” | Most of the patients agreed to remove the word 'bilateral' and just state ‘pain in both buttocks.’ |
| P3: “Dolor bilateral en los glúteos (bilateral button pain) is not a colloquial form of speech, but it can be understood. It is hard to imagine this pain if you have never experienced it.” | ||||
| P4: “’Bilateral’ is hard to understand. I understand that it means lateral. I would remove the word bilateral.” | ||||
| P6: “The word ‘bilateral’ is redundant because if the following question says that it can be in the right or left, then there is no need to use the term.’ The word 'bilateral' is too formal, I would only say ´pain in the buttocks´.” | ||||
| P7: “Not everyone knows what bilateral means. Better just to put ‘Dolor en los dos glúteos’ (pain in both buttocks)” | ||||
| P8: “Change the word’ bilateral’ for a synonym, for example, ‘in both buttocks.’” | ||||
| I3-Question | Have you ever had left and right buttock pain, at the same time or not? | ¿Ha tenido alguna vez dolor en el glúteo derecho o izquierdo, al mismo tiempo o por separado? | P1: “The text should specify more when this pain occurs (always, when sitting…). And replace the word ‘buttocks’ for upper part of the leg.” | It was not considered necessary to change this item. |
| P4: “I would simplify and say ‘Have you ever had pain in the buttocks at the same time or separately.’ I would remove reference to left or right.” | ||||
| I4-Title | Peripheral joint pain with swelling, before 50 years | Dolor articular periférico con hinchazón, antes de los 50 años | P1: “I would change peripheral for a more colloquial word such as ‘surrounding.’” | Most patients have difficulties understanding the meaning of ‘peripheral’. It is suggested to remove the term peripheral if in medical terms it does not provide any relevant information. |
| P3: “I am not sure what peripheral means. I understand it means pain the in the feet and hands” | ||||
| P5: “This would be more comprehensible if it didn’t use the word ‘peripheral.’” | ||||
| P6: “what is peripheral joint pain? Is it pain in the limbs? Explain what peripheral joint pain is or remove this expression. I have pain in my elbows, but I don’t know whether elbow pain is peripheral pain.” | ||||
| P7: “The term peripheral may be hard to understand. Perhaps I would remove peripheral from the expression.” | ||||
| P8: “External pain (the patient traces a circle with the fingers). Peripheral may be hard to understand. I would change to ‘external pain.’ Before 50 years of age, pain could be relative, because it may be pain unrelated to psoriatic arthritis.” | ||||
| I4-Question | Have you ever had a globally swollen and painful finger or toe? | ¿Ha tenido alguna vez hinchazón o dolor en una articulación? | P3: “It would be better to refer to inflammation rather than swelling.” | It was not considered necessary to change this item. |
| P4: “This is confusing because I have experienced pain, but not swelling before I was 50 years old. | ||||
| P6: “It might be a little confusing to refer to swelling or pain. Perhaps it could refer to swelling and/or pain?” |
Abbreviation: PURE-4, 4-item Psoriatic arthritis UnclutteRed screening Evaluation.
Comments made by the experts on the version with the patient suggestions were as follows:
- •
Item 1: no changes
- •
Item 2: the word inflamación (inflammation) was deleted as suggested by the patients.
- •
Item 3: no changes
- •
Item 4: at the end of the question, examples of joints have been added in parentheses (hands, feet, knees or ankles, for example).
Finally, after applying some grammatical corrections, the cultural adaptation of the PURE-4 questionnaire to Spanish was considered complete, obtaining a final translated version for administration to the Spanish population.
DiscussionThe present article describes the process followed for cultural adaptation of the PURE-4 questionnaire for use in the Spanish population with psoriasis.
By following a standard process,19 ensures the user of the questionnaire can be sure that the translation is not just literal; cultural aspects pertaining each item are taken into account to minimize errors due to interpretation of the content and to aid comprehension in patients of any cultural level. Thus, with this process, a version is obtained that is comparable with the original version in English. Some authors include variations in the process and recommendations to follow for cultural adaptation of instruments or questionnaires to measure PROs,21,22 but the basic and essential steps are, at the very least, forward translation with back translation, and cognitive debrief, following the standard process mentioned earlier. This is the process that was followed for adaptation of the PURE-4 questionnaire. In addition to maintaining the minimum steps for adaptation, a harmonization phase was performed throughout the process, rather than as an independent step.
It is essential that cultural adaptation of this type of questionnaire is performed correctly to avoid erroneous interpretations and ensure consistent results that enable both individual monitoring of patients and comparison of results with versions of the same questionnaire adapted to other countries. The methodology for cultural adaptation of a questionnaire should be followed appropriately so that, in the subsequent validation phase, the results are imputable only to the capacity for measuring the concept for which it was designed and not errors in the process of cultural adaptation.
During the process of cultural adaptation of the PURE-4 questionnaire to Spanish, difficulties were detected in the comprehension of some expressions or technical terms such as dactylitis or peripheral. These terms and expressions have therefore been adapted to facilitate comprehension and avoid erroneous interpretations. Given the way in which this adaptation was performed, it can be concluded that the questionnaire is fully comparable with the original version in terms of the content of the items.
One aspect to bear in mind is that this cultural adaptation is valid for the population whose mother tongue is Spanish as spoken in Spain. Additional checks would be needed to confirm that this same version is valid for example for Spanish spoken in South America.
ConclusionsTo date, the PURE-4 questionnaire has not been adapted linguistically and culturally to Spanish. Its adaptation is the first step for subsequent use in everyday clinical practice, and application of a standard methodology guarantees equivalence between the Spanish and original English versions.
This study has shown conceptual and linguistic equivalence of the Spanish version of the PURE-4 questionnaire adapted from the original English version.
FundingFunding for linguistic and cultural adaptation to Spanish of the PURE-4 questionnaire and for drafting of this manuscript was provided by Novartis Pharmaceuticals, España.
Conflicts of interestDr. Belinchón: has acted as consultant and/or speaker for and/or participated in clinical trials sponsored by companies that produce drugs for the treatment of psoriasis, including Janssen Pharmaceuticals Inc., Almirall, Lilly, AbbVie, Novartis, Celgene, Biogen Amgen, Leo-Pharma, Pfizer-Wyeth, UCB, and MSD.
Dr. Queiro: AbbVie, MSD, Pfizer, Novartis, Lilly, Janssen, UCB, and Celgene. He has received unrestricted research funds from Novartis, AbbVie, and Janssen. Consultant, investigator, and/or speaker.
Dr. Salgado-Boquete: AbbVie, Almirall, Celgene, Janssen, Leo, Lilly, Novartis, MSD, Pfizer, and Reig Jofre.
Dr. López-Ferrer: Novartis, Janssen, MSD, Lilly, Pfizer, Celgene, Almirall, Leo Pharma, AbbVie, and Amgen.
Dr. Ferran: has participated as speaker and/or consultant and/or participated in clinical trials for Janssen, Lilly, Novartis, Pfizer, MSD, AbbVie, Celgene, and Almirall.
Dr. Coto-Segura: potential conflicts of interest (steering committee member, consultant, subsidies, investigation support, participation in clinical trials, and speaker fees) with the following pharmaceutical companies: AbbVie (Abbott), Janssen-Cilag, Novartis, Pfizer, MSD, UCB pharma, Lilly, and Celgene.
Dr. Rivera: AbbVie, Almirall, Celgene Corporation, GSK, Janssen-Cilag, Lilly, LEO Pharma, MSD, Novartis, Pfizer, and UCB. Consultant, investigator, and/or speaker.
Dr. Vidal: Lilly, Janssen, AbbVie, Novartis, UCB, Celgene, Gebro, and Leo. Consultant, investigator, and/or speaker.
Dr. Rodríguez: consultant and speaker for AbbVie, Janssen Pharmaceuticals Inc., MSD, Pfizer-Wyeth, Novartis, Celgene, Almirall SA, Lilly, and Leo-Pharma.
Dr. de la Cueva: consultant and/or speaker and/or participant in clinical trials sponsored by companies such as AbbVie, Almirall, Amgen, Boehringer, Biogen, Celgene, Gebro, Janssen Cilag, Leo-Pharma, Lilly, MSD, Novartis, Pfizer- Wyeth, Sandoz, Sanofi, and UCB.
Dr. Guinea and Dr. Martín Vázquez: employees of Novartis.
We would like to thank the Acción Psoriasis (Psoriasis Action) patient group for their collaboration during the study.
We thank IQVI and Carmen Barrull and Núria Perulero for providing medical editorial assistance with this manuscript.
Please cite this article as: Belinchón I, Queiro R, Salgado-Boquete L, López-Ferrer A, Ferran M, Coto-Segura P, et al. Adaptación lingüística y cultural al español del cuestionario para Psoriatic arthritis UnclutteRed screening Evaluation (PURE-4). Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.03.004