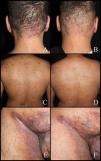
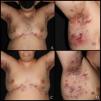
Hidradenitis suppurativa (HS) is a chronic, inflammatory, and recurrent disease characterized by painful deep nodules, abscesses, and sinus tracts in skin areas with a high density of apocrine glands (axillae, groin, perianal and perineal regions, etc.). Patients’ quality of life is threatened by pain, drainage, foul odor, and medical complications they may experience.1
Different lines of treatment exist depending on the extent and severity of HS. Among them, topical or systemic antibiotics are the most widely used drugs due to their anti-inflammatory and antimicrobial properties. Although their use remains controversial, their activity and efficacy have been demonstrated in multiple studies.1–4 In mild-to-moderate forms of the disease, topical and oral clindamycin, as well as tetracyclines and rifampicin, may be used. In more severe forms, combinations of these drugs may be required, often for long periods of time.1,5 Since 2016, multiple studies have demonstrated the efficacy of IV ertapenem in the treatment of recalcitrant HS.6 However, this antibiotic presents several drawbacks, such as the need for daily intravenous administration and its contraindication in patients allergic to β-lactams. Our article demonstrates the efficacy of linezolid in patients with severe HS, providing a new therapeutic alternative for these individuals.
We describe a retrospective case series of 6 patients, consisting of 5 men and 1 girl, treated with linezolid (Table 1). All cases exhibited recalcitrant Hurley stage III HS and had a mean International Hidradenitis Suppurativa Severity Score System (IHS4) of 19 (range, 12–26). Patients’ ages ranged from 13 to 75 years, with a median of 40,5 years. Comorbidities included advanced-stage pancreatic cancer (case #4) and psoriasis (case #5). Additionally, 2 patients were allergic to β-lactams, which limited the use of these antibiotics. Three patients were smokers.
Clinical and demographic characteristics of patients treated with linezolid.
| Case | A/S | β-Lactam allergy | Comorbidities | Hurley stage | Locations | Duration (weeks) | CRP pre-treatment (mg/L) | CRP post-treatment (mg/L) | Maintenance biologic | IHS4 pre-treatment | IHS4 post-treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case #1 | 43/M | No | Smoker, acne | III | Occipital, inguinal, perineal, perianal, scrotal | 6 | 60 | 3 | Infliximab 10mg/kg | 24 | 8 |
| Case #2 | 74/M | No | Smoker and drinker, latent TB, Buerger disease | III | Inguinal | 6 | 38 | 12 | Infliximab 10mg/kg | 22 | 11 |
| Case #3 | 25/M | Yes | Acne | III | Axillary, inframammary | 2 | 61 | 5 | Infliximab 7.5mg/kg | 26 | 13 |
| Case #4 | 65/M | No | Stage IV pancreatic cancer, latent TB, psoriasis, smoker | III | Inguinal | 6 | 45 | 26 | No | 14 | 4 |
| Case #5 | 38/M | Yes | Psoriasis | III | Inguinal | 1 | 86 | 3 | Infliximab 7.5mg/kg | 16 | 8 |
| Case #6 | 13/F | No | Overweight | III | Axillary, inguinal | 2 | 3.9 | 1.1 | Infliximab 7.5mg/kg | 12 | 6 |
A: age; S: sex; IHS4: International Hidradenitis Suppurativa Severity Score System; CRP: C-reactive protein; TB: tuberculosis.
Patients received linezolid 600mg every 12h for treatment durations ranging from 1 to 6 weeks, with longer treatment intervals in patients with more severe disease. Five of the patients were on maintenance biologic therapy with infliximab 7.5–10mg/kg every 2 weeks. One patient received concomitant treatment with acitretin and metronidazole, and another with dapsone. In 4 patients, linezolid was not associated with any concomitant treatments besides maintenance infliximab. One patient died due to progression of his underlying condition (case #4). After treatment completion, all patients experienced clinical improvement, achieving at least a 50% reduction in IHS4 (Figs. 1 and 2). Furthermore, 5 of the 6 cases showed a decrease in inflammatory parameters.
Linezolid is an oxazolidinone that acts by inhibiting protein synthesis. It is a bacteriostatic antibiotic effective against methicillin-resistant Staphylococcus aureus and can be administered orally as well as intravenously, allowing outpatient use. It may be used in patients of all ages and in individuals with impaired hepatic or renal function. Although considered safe and well tolerated, its long-term use has been associated with more severe adverse effects such as reversible myelosuppression, mainly in the form of thrombocytopenia. It may also cause reversible retinopathy after drug withdrawal, irreversible peripheral neuropathy, and lactic acidosis. It is contraindicated in patients on MAOi (monoamine oxidase inhibitors) or SSRIs (selective serotonin reuptake inhibitors) due to the risk of serotonin syndrome.6
Ertapenem, on the other hand, is a broad-spectrum β-lactam antibiotic. In HS patients, it is used in cases refractory to other treatments or when surgery is contraindicated. As previously mentioned, treatment is administered intravenously and must be prolonged between 6 and 16 weeks, depending on disease severity, to avoid early relapse.7,8
In the management of HS, linezolid offers several advantages over ertapenem. Its oral administration makes it a more convenient treatment option. Additionally, its usability in patients allergic to β-lactams makes it a reliable alternative in these cases. As a disadvantage, linezolid is considered a less safe treatment due to the adverse effects mentioned above. In the literature, there is only 1 reported case of a patient with Hurley stage III HS treated intravenously with linezolid plus meropenem, with significant improvement.9 Our series describes the first 6 cases of patients treated with oral linezolid, with none of them experiencing adverse effects. This demonstrates its efficacy in patients with severe HS who are on biologic treatment and refractory to other antibiotic therapies.
In conclusion, we propose linezolid as a convenient and effective therapeutic alternative, particularly in cases where the use of other antibiotics is contraindicated.
Conflict of interestThe authors declare that they have no conflict of interest.