Interferons (IFN) are a family of pleiotropic cytokines that exert antiviral and antitumor effects through several different mechanisms (Table 1).1 These cytokines are of interest to dermatologists mainly because of their effectiveness in the treatment of basal cell carcinoma (BCC), squamous cell carcinoma, Kaposi sarcoma, and melanoma.2
Interferons: Indications and Mechanisms of Action.
| Indications |
| Antiviral |
| Hepatitis Ba,b |
| Hepatitis Ca,b |
| Human papilloma virusa,c |
| Antitumor |
| Hepatocarcinomab |
| Chronic myeloid leukemia,a,b hairy cell leukemia,a,b multiple myelomab |
| Kaposi sarcomaa,b |
| Renal carcinomaa |
| Basal cell carcinomab |
| Squamous cell carcinomab |
| Melanomaa,b |
| Mechanisms of action |
| Suppression of cell proliferation |
| Increased macrophage-mediated phagocytosis |
| Inhibition of viral replication |
| Inhibition of angiogenesis |
| Increase in cellular immune response of T lymphocytes |
Interferon subtype: a, alfa-2a; b, alfa-2b; c, alfa-n3.
Intralesional IFN was first demonstrated to effectively treat BCC in 1986,3 and it produced complete responses in between 67% and 80% of patients in published series. IFN can be administered as monotherapy or as an adjuvant after surgery. Here, we describe the use of IFN administered topically in ophthalmic eyedrops in the management of a BCC on the free margin of the eyelid. This formulation is used in ophthalmology to treat squamous conjunctival papillomata,4 squamous neoplasms of the ocular surface, Kaposi sarcomas, and conjunctival melanomas.5
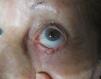
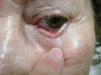
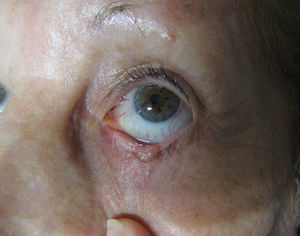
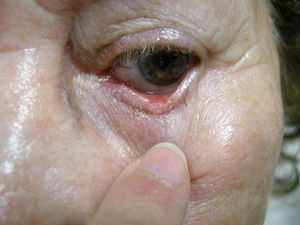
We report the case of an 88 year-old woman with a histologically-confirmed, solid papular BCC of 5mm in diameter on the margin of the lower left eyelid (Fig. 1). The patient refused surgical treatment. Other treatment options, such as photodynamic therapy and imiquimod cream, were considered, but were ruled out due to the characteristics and location of the lesion. The patient also refused treatment with intralesional IFN due to a fear of injections. It was decided to treat the BCC with IFN alfa-2b in ophthalmic eyedrops at a concentration of 1 million IU/mL, administered 4 times per day.6,7 This treatment was continued for 4 months, resulting in a decrease in the size of the lesion (Fig. 2). No adverse effects were observed during treatment, and there was no change in the clinical appearance of the lesion on follow-up at 39 months. The patient still refuses to undergo either surgery or a control biopsy.
The efficacy of IFN alfa-2b eyedrops in various tumors of the conjunctiva, eyelid, and ocular surface is described in the ophthalmic literature.2,5,7,8 The recommended dose is 1 drop of IFN alfa-2b at 1 million IU/mL 4 times per day for 3 to 4 months. Some authors recommend a maintenance regimen of 1 drop every 12hours.6 Several studies have compared ophthalmic with intralesional IFN alfa-2b administration8 in the treatment of both non-invasive and invasive ocular surface squamous neoplasia, reporting better patient compliance and lower rates of local and systemic side effects in patients treated with eyedrops. The only local side effects reported are mild and resolve upon discontinuation of treatment. They include punctate keratitis, follicular conjunctivitis, and conjunctival hyperemia.5,8,9 Development of the flu syndrome characteristic of systemic or intralesional IFN therapy is rare.6,7 Comparison of IFN alfa-2b eyedrops with surgical treatment of non-invasive squamous neoplasia has revealed a comparable cure rate (total resolution in 96.4% of patients), with eyedrops producing better cosmetic results and less destruction of limbal stem cells.10
While we found no mention of this treatment in the dermatological literature, several ophthalmologic publications describe the effectiveness of topical IFN alfa-2b in cases of viral warts, intraepidermal carcinomas, and even melanomas on the eyelids and ocular surface, although these results are described in small series or isolated cases and should thus be interpreted with caution. We found no other cases in which IFN alfa-2b eyedrops have been used in the management of BCC of the eyelid. BCC is an accepted indication for IFN alfa-2b, and our findings point to a new potential route of administration. In our case the volume of the tumor decreased considerably, and the patient remains clinically stable after 3 years. However, we have no objective evidence of resolution, which requires close monitoring in a clinical setting. Our isolated experience should in no way change the standard accepted approaches used for the management of nonmelanoma skin cancer. Controlled clinical trials will be necessary to definitively determine the effectiveness of this treatment. However, given its ease of administration and its few, mild side effects, we propose the use of IFN alfa-2b eyedrops as a neoadjuvant therapy in selected cases to reduce tumor size before microscopically controlled surgical excision.
Please cite this article as: Leis-Dosil VM, Prats-Caelles I, Rubio-Flores C. Colirio de interferón y carcinoma basocelular palpebral. Actas Dermosifiliogr. 2014;105:207–208.