The JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathway is an essential final step in the signaling process of most interleukins with a critical role in the pathogenesis of atopic dermatitis. By achieving broad, intermittent inhibition of the activity of multiple cytokines, JAK inhibitors help to modulate T helper 2 cell-mediated inflammation, epidermal barrier dysfunction, and itch signaling. This comprehensive blockade, however, can result in a wider range of adverse effects. We review a number of JAK inhibitors that have been recently approved for use in atopic dermatitis, such as baricitinib, upadacitinib, and abrocitinib, as well as others that are currently in the pipeline or under development, such as gusacitinib, delgocitinib, ruxolitinib, brepocitinib, tofacitinib, and cerdulatinib. The use of JAK inhibitors to block the signaling of numerous cytokines with a critical role in the pathogenesis of atopic dermatitis has revolutionized the treatment of this pathogenically complex, phenotypically heterogeneous skin disease.
La vía Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) es esencial en la señalización final de una gran mayoría de interleucinas (IL) fundamentales en la patogénesis de la dermatitis atópica (DA). El bloqueo transversal que consiguen los inhibidores de JAK a través de la inhibición intermitente de las acciones de múltiples citoquinas, permite modular la inflamación Th2, la disfunción de barrera epidérmica y la señalización del prurito. Sin embargo, esa inhibición amplia también puede asociarse con una mayor variedad de efectos adversos. En este artículo se revisan los inhibidores de JAK recientemente aprobados en la DA —baricitinib, upadacitinib y abrocitinib—, así como otros emergentes o en desarrollo como gusacitinib, delgocitinib, ruxolitinib, brepocitinib, tofacitinib y cerdulatinib. El bloqueo de la señalización de diversas citoquinas relevantes en esta dermatosis, compleja patogénicamente y con una expresión fenotípica heterogénea, a través de los inhibidores de JAK, ha supuesto una revolución en el tratamiento de la DA.
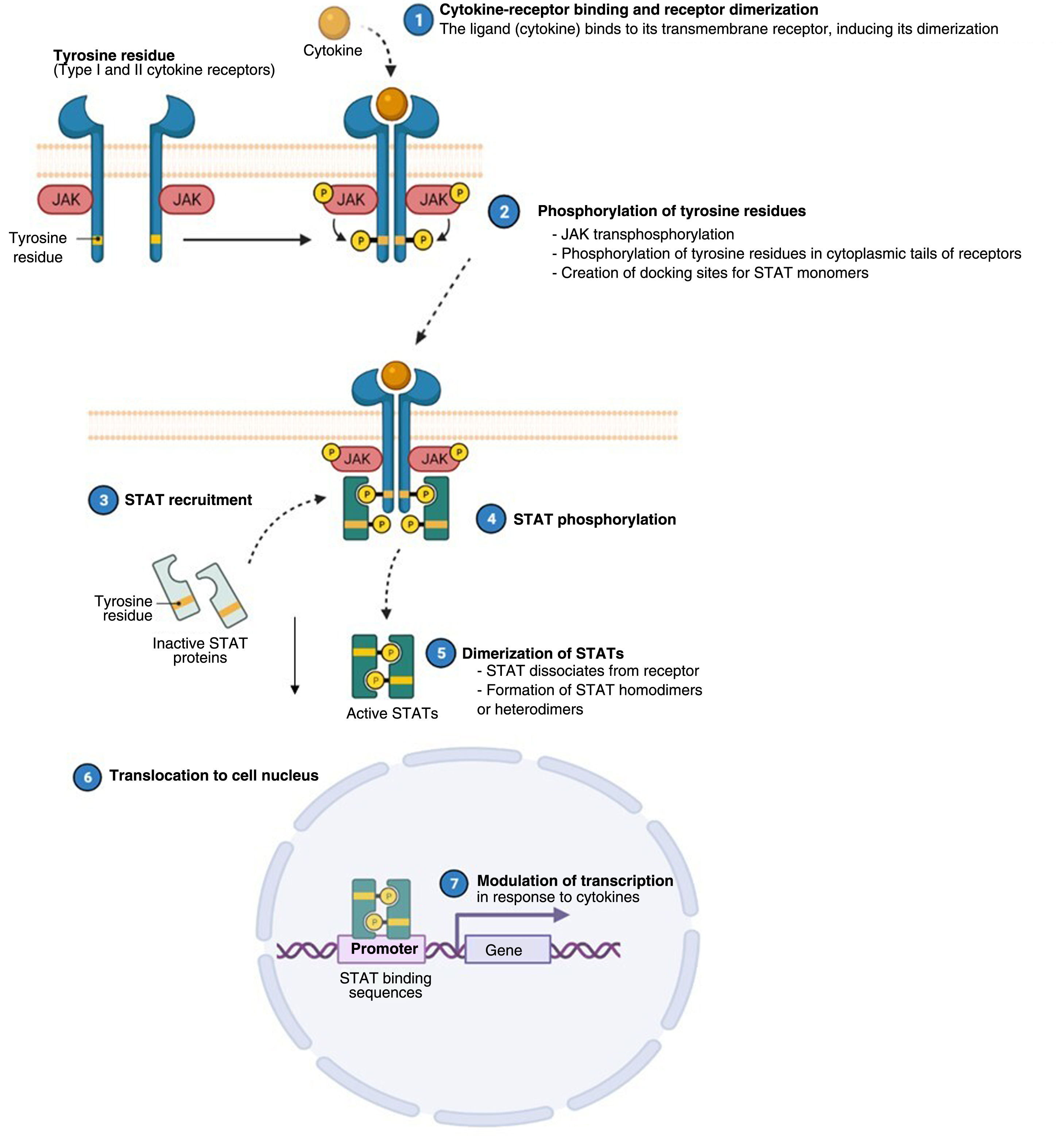
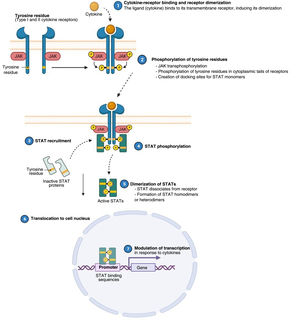
Atopic dermatitis (AD) is a chronic, relapsing inflammatory disease1 associated with high morbidity.2 It has variable clinical presentations linked to diverse underlying pathophysiologic mechanisms and interactions between genetic predisposition, environmental factors, and immune dysregulation.3 Over the past decade, significant advances in our understanding of the pathogenesis of AD have led to the development of new therapies.4 Inflammation in AD is predominantly mediated by a type 2 T helper (TH2) response involving interleukin (IL) 4, IL-13, IL-33, and IL-31. This last IL is a potent mediator of itch. IL-17, IL-22, and interferon (IFN) γ also have an important role in chronic stages of the disease. In order to act on cells, most cytokines involved in AD have to rely on their transmembrane cytokine receptor (type I or II). Following cytokine-receptor binding, 2 JAK isoforms (as homodimers or heterodimers) are autophosphorylated, activating signal transducers and activators of transcription (STATs), which are finally translocated to the nucleus where they modulate the transcription of target genes.3,5
Approval of the first biologic for use in AD, dupilumab, which is directed against the alpha subunit of the IL-4 receptor, represented a major step forward in the treatment of this disease.6 Other breakthroughs include the recent approval of tralokinumab, an antibody against IL-13, and the development of other monoclonal antibodies, now at a very advanced stage.7 These include lebrikizumab, an IL-13 signaling inhibitor, which has produced promising results in terms of improving the signs and symptoms of AD,8,9 and nemolizumab, an anti-IL-31 antibody, which achieves a rapid and sustained reduction in itch, although with less significant improvements compared with novel treatments already available.10,11 Considering the variable clinical spectrum of AD, even within individual patients, probably linked to the wide range of cytokines involved in the pathogenesis of this disease, there is growing interest in the use of Janus kinase (JAK) inhibitors, as these can simultaneously interfere with the signaling of multiple cytokines.
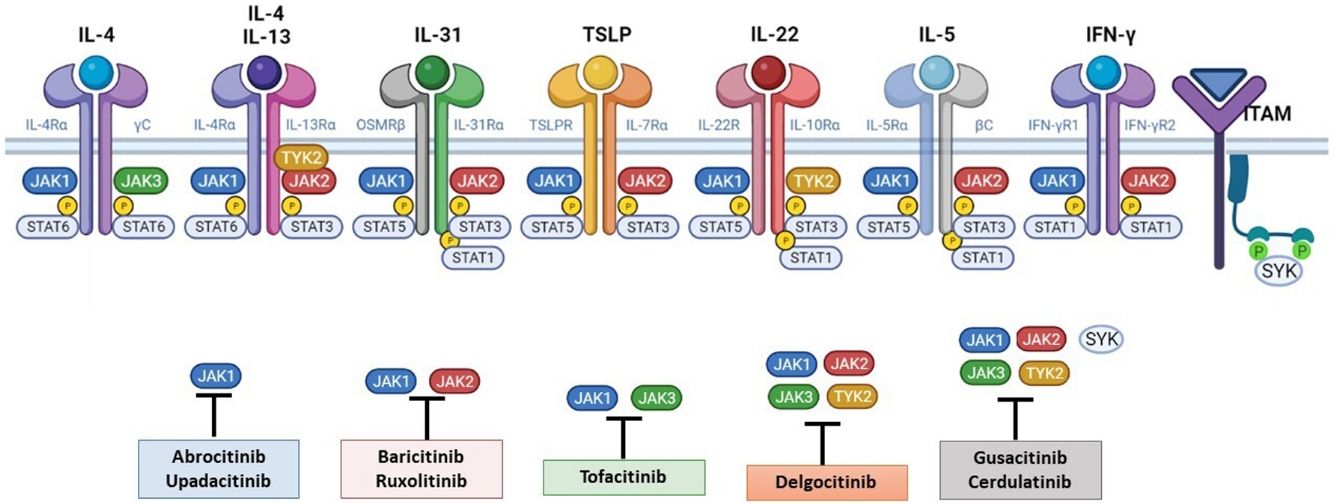
The Role of the JAK-STAT Pathway in the Pathogenesis of ADStructure and Function of the JAK-STAT Signaling PathwayA number of molecules that have proven effective in AD have been developed in recent years. They act by modulating the activity of the JAK/STAT signaling pathway, which has a fundamental role in both innate and adaptive immunity.12 As mentioned, the cytokines involved in AD rely on their transmembrane receptor (type I or II cytokine receptors) to exert their effects on cells. Because the receptors do not have intrinsic tyrosine kinase activity, they in turn require the action of JAK enzymes (Table 1).13,14 Four JAKs – JAK1, JAK2, JAK3, and TYK2 – have been identified in human cells. They form heterodimers and heterotrimers with each other, and JAK2 can additionally form homodimers (Fig. 1).12,15 Each cytokine receptor recruits and uses a particular combination of JAKs. This has implications for JAK inhibition in a range of immune-mediated diseases and cancers.16
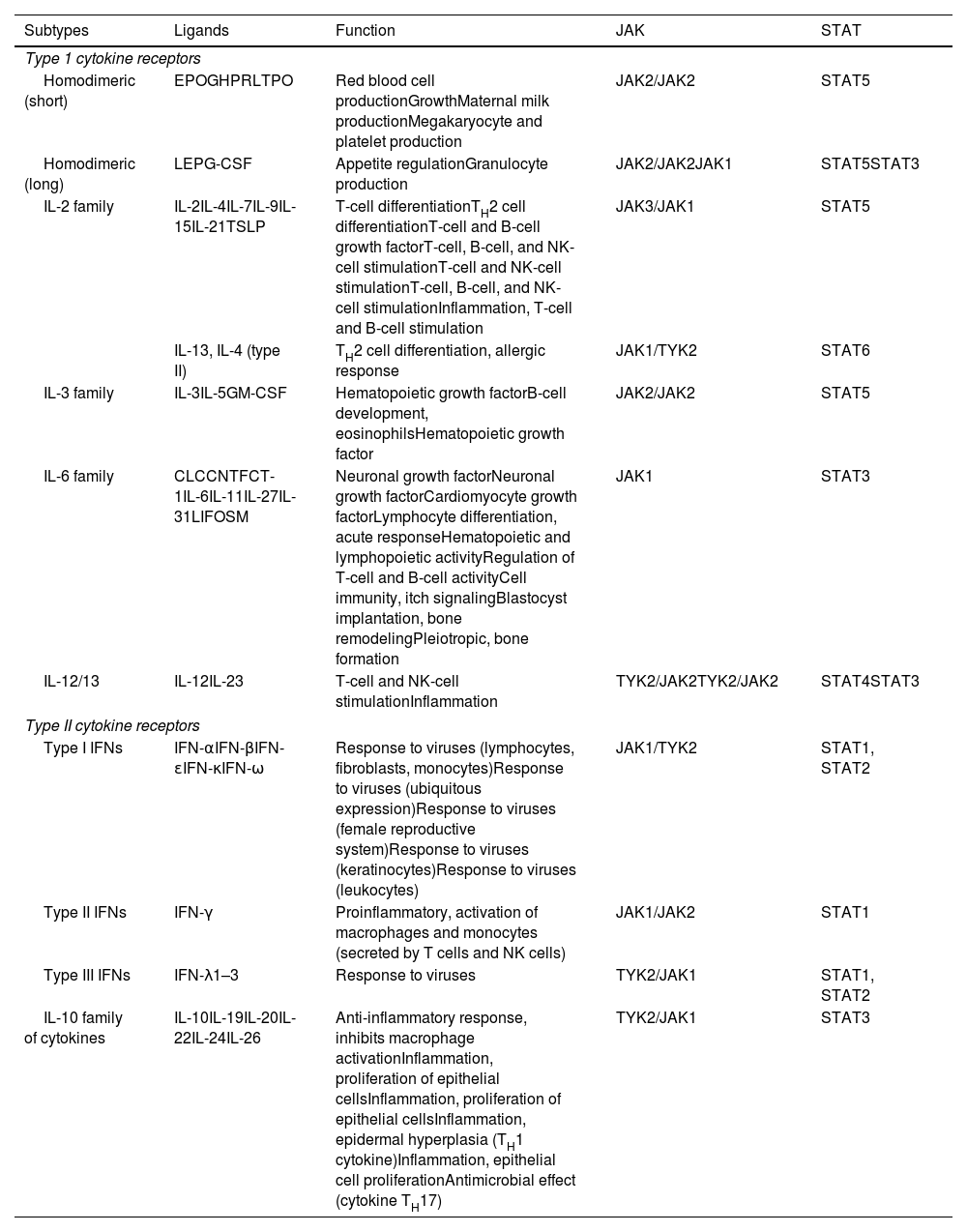
Type I and II Cytokine Receptors.
| Subtypes | Ligands | Function | JAK | STAT |
|---|---|---|---|---|
| Type 1 cytokine receptors | ||||
| Homodimeric (short) | EPOGHPRLTPO | Red blood cell productionGrowthMaternal milk productionMegakaryocyte and platelet production | JAK2/JAK2 | STAT5 |
| Homodimeric (long) | LEPG-CSF | Appetite regulationGranulocyte production | JAK2/JAK2JAK1 | STAT5STAT3 |
| IL-2 family | IL-2IL-4IL-7IL-9IL-15IL-21TSLP | T-cell differentiationTH2 cell differentiationT-cell and B-cell growth factorT-cell, B-cell, and NK-cell stimulationT-cell and NK-cell stimulationT-cell, B-cell, and NK-cell stimulationInflammation, T-cell and B-cell stimulation | JAK3/JAK1 | STAT5 |
| IL-13, IL-4 (type II) | TH2 cell differentiation, allergic response | JAK1/TYK2 | STAT6 | |
| IL-3 family | IL-3IL-5GM-CSF | Hematopoietic growth factorB-cell development, eosinophilsHematopoietic growth factor | JAK2/JAK2 | STAT5 |
| IL-6 family | CLCCNTFCT-1IL-6IL-11IL-27IL-31LIFOSM | Neuronal growth factorNeuronal growth factorCardiomyocyte growth factorLymphocyte differentiation, acute responseHematopoietic and lymphopoietic activityRegulation of T-cell and B-cell activityCell immunity, itch signalingBlastocyst implantation, bone remodelingPleiotropic, bone formation | JAK1 | STAT3 |
| IL-12/13 | IL-12IL-23 | T-cell and NK-cell stimulationInflammation | TYK2/JAK2TYK2/JAK2 | STAT4STAT3 |
| Type II cytokine receptors | ||||
| Type I IFNs | IFN-αIFN-βIFN-ɛIFN-κIFN-ω | Response to viruses (lymphocytes, fibroblasts, monocytes)Response to viruses (ubiquitous expression)Response to viruses (female reproductive system)Response to viruses (keratinocytes)Response to viruses (leukocytes) | JAK1/TYK2 | STAT1, STAT2 |
| Type II IFNs | IFN-γ | Proinflammatory, activation of macrophages and monocytes (secreted by T cells and NK cells) | JAK1/JAK2 | STAT1 |
| Type III IFNs | IFN-λ1–3 | Response to viruses | TYK2/JAK1 | STAT1, STAT2 |
| IL-10 family of cytokines | IL-10IL-19IL-20IL-22IL-24IL-26 | Anti-inflammatory response, inhibits macrophage activationInflammation, proliferation of epithelial cellsInflammation, proliferation of epithelial cellsInflammation, epidermal hyperplasia (TH1 cytokine)Inflammation, epithelial cell proliferationAntimicrobial effect (cytokine TH17) | TYK2/JAK1 | STAT3 |
Type I and II cytokine receptors. The table shows the different families of cytokines and their receptors and the JAK/STAT signaling pathways used to exert their effects.
Abbreviations, CLC, cardiotrophin-like cytokine; CNTF, ciliary neurotrophic growth factor; CT1, cardiotrophin 1; EPO, erythropoietin; G-CSF, granulocyte colony-stimulating factor; GH, growth hormone; GM-CSF, granulocyte/macrophage colony stimulating factor; IFN, interferon; JAK, Janus kinase; LEP, leptin; LIF, leukemia inhibitory factor; NK, natural killer; OSM, oncostatin M; PRL, prolactin; STAT, signal transducer and activator of transcription; TPO, thrombopoietin; TSLP, thymic stromal lymphopoietin; TYK2, tyrosine kinase 2.
Four JAKs have been identified in human cells: JAK1, JAK2, JAK3, and TYK2. These form heterodimers and heterotrimers amongst themselves; JAK2 in addition can form homodimers. There are 7 types of STATs (STATs 1–6, including the homologs STAT5a and STAT5b). More than 50 cytokines and growth factors have been identified in this signaling pathway, including interferons, cytokines, hormones, and colony-stimulating factors. Downstream JAK/STAT-mediated events include hematopoiesis, tissue repair, immune function, inflammation, apoptosis, and adipogenesis. JAKs noncovalently associate with cytokine receptors and mediate cytokine receptor phosphorylation and the recruitment of 1 or more STAT proteins. STATs in their phosphorylated form subsequently dimerize, forming homodimers or heterodimers, and are translocated to the nucleus, where they bind to specific sequences of the promoter and regulate the expression of various target genes according to the action of each cytokine. JAK indicates Janus kinase; STAT, signal transducer and activator of transcription; TYK, tyrosine kinase 2. Source: Figure adapted from the figure “Cytokine Signaling through the JAK-STAT Pathway” produced at BioRender.com (2023). Obtained from https://app.biorender.com/biorender-templates/figures/all/t-5fac3e99614e0c00aac4a356-cytokine-signaling-through-the-jak-stat-pathway.
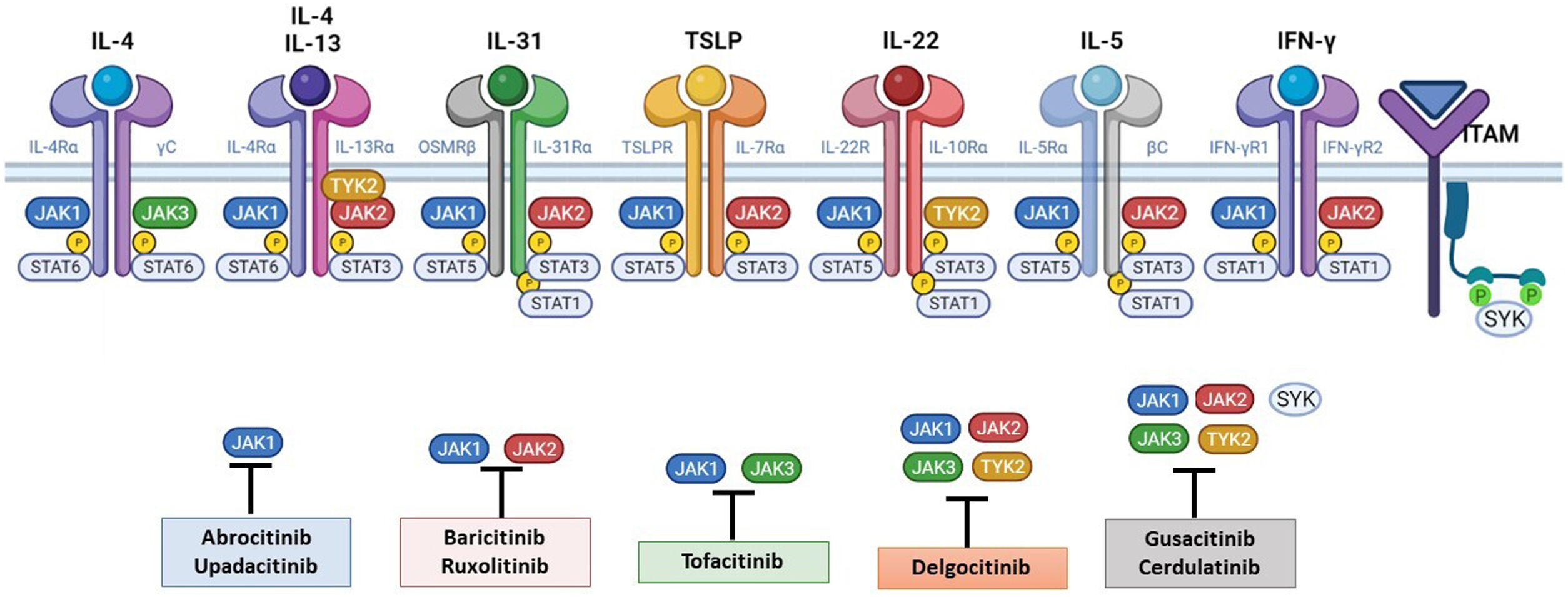
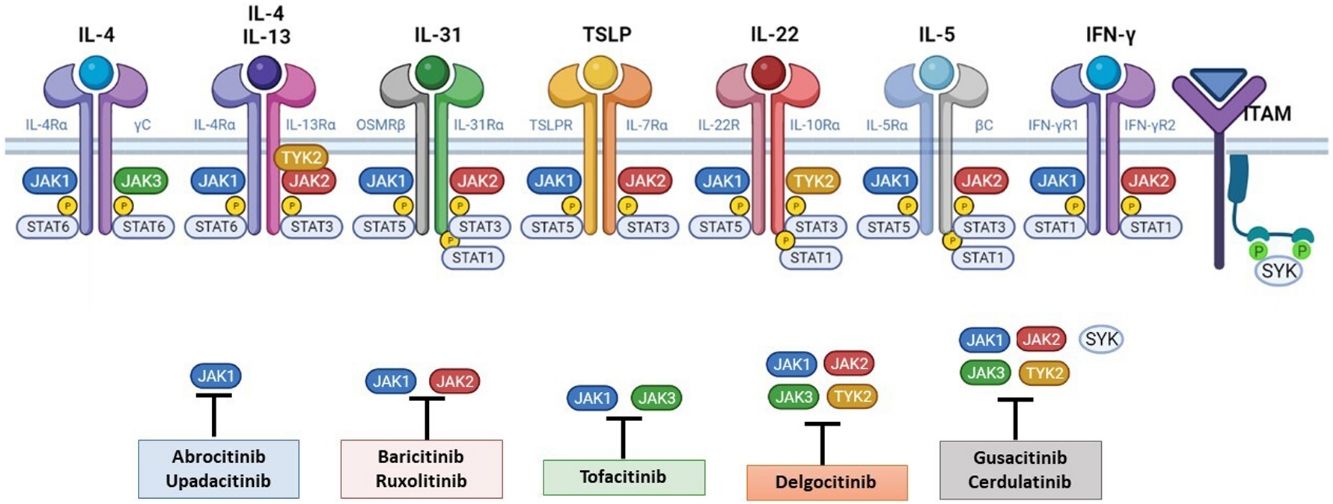
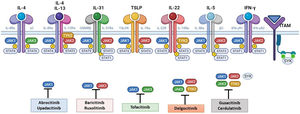
The pathogenesis of AD is complex and involves several intersecting factors. The TH2-mediated immune response is crucial in AD, and the JAK/STAT pathway is involved in the signaling of TH2 cytokines (IL-4, IL-13, IL-31). IL-4 has 2 types of receptors. Type I is formed by the IL-4Rα chain and the common γ chain and its signaling is mediated through JAK1/JAK3 with subsequent activation of STAT6. Type II is formed by the IL-4Rα chain and the IL-13Rα1 chain and induces the activation of JAK1 and TYTK2 and the downstream activation of transcription factors STAT6 and STAT3. STAT6 is essential to the final function of IL-4 and IL-13. IL-31 exerts its effects on the IL-31 receptor (formed by the IL-31Rα chain and the β chain of the oncostatin M receptor) through the JAK/STAT pathway, the protein kinase B pathway, and the mitogen-activated protein kinase pathway. JAK/STAT signaling also drives itch, the main symptom of AD.17
Although TH2-mediated responses have a central role in AD, responses mediated by TH1, TH17, and TH22 are variably involved in more chronic stages of the disease and are more common in certain phenotypes.4 IL-22, for example, which is a member of the IL-10 family, has an essential role in epidermal hyperplasia. Its binding to the IL-22 receptor (IL-22R), which is composed of the IL-22R1 and IL-10R2 chains, induces activation of JAK1 and TYK2, ultimately leading to STAT3 activation (Fig. 2). IL-17 signaling, by contrast, does not depend on the JAK/STAT pathway.
Main cytokine receptors and the JAK/STAT signaling pathway. For the cytokines involved in atopic dermatitis to act on cells, they must bind to their transmembrane receptor, which requires the action of cytoplasmic JAK enzymes. JAK/STAT signaling is thus key in the pathogenesis of this disease. JAK inhibition enables simultaneous inhibition of multiple cytokines. Some JAK inhibitors are more selective (inhibiting certain JAKs), while others can inhibit all isoforms. IFN indicates interferon; IL, interleukin; ITAM, immunoreceptor tyrosine-based activation motif; JAK, Janus kinase; STAT, signal transducer and activator of transcription; SYK, spleen tyrosine kinase; TSLP, thymic stromal lymphopoietin; TYK, tyrosine kinase 2. Source: Figure created at BioRender.com.
Biologics are used in the treatment of AD in order to achieve a tailored approach, whereby certain cytokines are prevented from binding to their receptors. Considering that the JAK/STAT pathway is the signaling pathway for these receptors, JAK inhibitors may offer therapeutic benefits in this setting.
The JAK-STAT pathway is a paradigm of rapid membrane-to-nucleus signaling, and JAK inhibitors appear to be associated with relatively rapid kinetic responses compared with monoclonal antibodies.18 There does appear, however, to be certain immunologic redundancy in the JAK-STAT system, as there are more than 50 cytokines but just 4 JAK isoforms. The pathway thus participates in convergent immunologic mechanisms, meaning less selective inhibition.19 JAK inhibitors are small molecules, administered orally or topically, that interfere in a broader, albeit intermittent, way with the cytokines involved in AD; their effectiveness also depends on the selectivity of the drug (greater or less inhibition).16,20
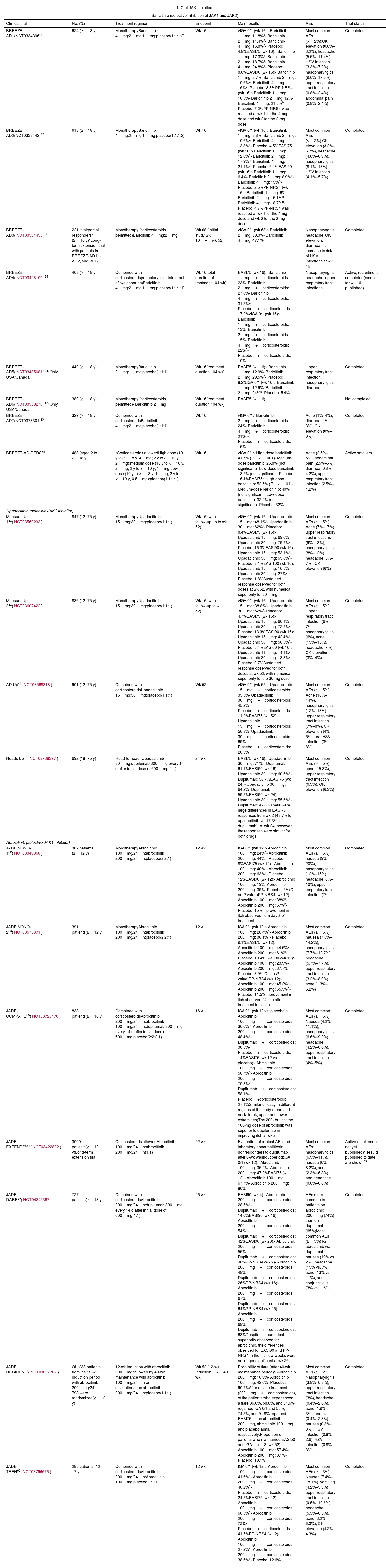
Oral JAK InhibitorsIn this next section, we look at the range of systemic JAK inhibitors that have either been recently authorized or are in the process of being authorized for the treatment of moderate to severe AD (Table 2).
Results of the Main Clinical Trials on the Use of JAK Inhibitors in Atopic Dermatitis.
| 1. Oral JAK inhibitors | ||||||
|---|---|---|---|---|---|---|
| Baricitinib (selective inhibition of JAK1 and JAK2) | ||||||
| Clinical trial | No. (%) | Treatment regimen | Endpoint | Main results | AEs | Trial status |
| BREEZE-AD1(NCT0334396)27 | 624 (≥18 y) | MonotherapyBaricitinib 4mg:2mg:1mg:placebo(1:1:1:2) | Wk 16 | vIGA 0/1 (wk 16):- Baricitinib 1mg: 11.8%a- Baricitinib 2mg: 11.4%a- Baricitinib 4mg: 16.8%b- Placebo: 4.8%EASI75 (wk 16):- Baricitinib 1mg: 17.3%a- Baricitinib 2mg: 18.7%d- Baricitinib 4mg: 24.8%b- Placebo: 8.8%EASI90 (wk 16):- Baricitinib 1mg: 8.7%- Baricitinib 2mg: 10.6%a- Baricitinib 4mg: 16%b- Placebo: 8.8%PP-NRS4 (wk 16):- Baricitinib 1mg: 10.5%- Baricitinib 2mg: 12%- Baricitinib 4mg: 21.5%b- Placebo: 7.2%PP-NRS4 was reached at wk 1 for the 4-mg dose and wk 2 for the 2-mg dose. | Most common AEs (>2%):CK elevation (0.8%–3.2%), headache (5.5%–11.4%), HSV infection (3.3%–7.2%), nasopharyngitis (9.6%–17.3%), upper respiratory tract infection (0.8%–2.4%), abdominal pain (0.8%–2.4%) | Completed |
| BREEZE-AD2(NCT0333442)27 | 615 (≥18 y) | MonotherapyBaricitinib 4mg:2mg:1mg:placebo(1:1:1:2) | Wk 16 | vIGA 0/1 (wk 16):- Baricitinib 1mg: 8.8%- Baricitinib 2mg: 10.6%a- Baricitinib 4mg: 13.8%d- Placebo: 4.5%EASI75 (wk 16):- Baricitinib 1mg: 12.8%a- Baricitinib 2mg: 17.9%b- Baricitinib 4mg: 21.1%b- Placebo: 6.1%EASI90 (wk 16):- Baricitinib 1mg: 6.4%- Baricitinib 2mg: 8.9%d- Baricitinib 4mg: 13%b- Placebo: 2.5%PP-NRS4 (wk 16):- Baricitinib 1mg: 6%- Baricitinib 2mg: 15.1%d- Baricitinib 4mg: 18.7%b- Placebo: 4.7%PP-NRS4 was reached at wk 1 for the 4-mg dose and wk 2 for the 2-mg dose. | Most common AEs (>2%):CK elevation (3.2%–5.7%), headache (4.8%–8.9%), nasopharyngitis (8.1%–13%), HSV infection (4.1%–5.7%) | Completed |
| BREEZE-AD3(NCT03334435)26 | 221 total/partial responders* (≥18 y)*Long-term extension trial with patients from BREEZE-AD1, -AD2, and -AD7 | Monotherapy (corticosteroids permitted)Baricitinib 4mg:2mg | Wk 68 (initial study wk 16+wk 52) | vIGA 0/1 (wk 68):- Baricitinib 2mg: 59.3%- Baricitinib 4mg: 47.1% | Nasopharyngitis, headache, CK elevation, diarrhea; no increase in risk of HSV infections at wk 16 | Completed |
| BREEZE-AD4(NCT03428100)25 | 463 (≥18 y) | Combined with corticosteroids(refractory to or intolerant of cyclosporine)Baricitinib 4mg:2mg:1mg:placebo(1:1:1:1) | Wk 16(total duration of treatment 104 wk) | EASI75 (wk 16):- Baricitinib 1mg+corticosteroids: 23%- Baricitinib 2mg+corticosteroids: 27.6%- Baricitinib 4mg+corticosteroids: 31.5%a- Placebo+corticosteroids: 17.2%vIGA 0/1 (wk 16):- Baricitinib 1mg+corticosteroids: 13%- Baricitinib 2mg+corticosteroids: 15%- Baricitinib 4mg+corticosteroids: 22%a- Placebo+corticosteroids: 10% | Nasopharyngitis, headache, upper respiratory tract infections | Active, recruitment completed(results for wk 16 published) |
| BREEZE-AD5(NCT03435081)24*Only USA/Canada | 440 (≥18 y) | MonotherapyBaricitinib 2mg:1mg:placebo(1:1:1) | Wk 16(treatment duration 104 wk) | EASI75 (wk 16):- Baricitinib 1mg: 12.9%- Baricitinib 2mg: 29.5%b- Placebo: 8.2%IGA 0/1 (wk 16):- Baricitinib 1mg: 12.9%- Baricitinib 2mg: 24%b- Placebo: 5.4% | Upper respiratory tract infection, nasopharyngitis, diarrhea | Completed |
| BREEZE-AD6(NCT03559270)17*Only USA/Canada | 380 (≥18 y) | Monotherapy (corticosteroids permitted)- Baricitinib 2mg | Wk 16(treatment duration 104 wk) | EASI75 (wk 16) | Not completed | |
| BREEZE-AD7(NCT0373301)23 | 329 (≥18 y) | Combined with corticosteroidsBaricitinib 4mg:2mg:placebo(1:1:1) | Wk 16 | vIGA 0/1:- Baricitinib 2mg+corticosteroids: 24%- Baricitinib 4mg+corticosteroids: 31%d- Placebo+corticosteroids: 15% | Acne (1%–4%), diarrhea (1%–3%), CK elevation (0%–3%) | Completed |
| BREEZE-AD-PEDS35 | 483 (aged 2 to <18 y) | *Corticosteroids allowedHigh dose (10 y to <18 y, 4mg; 2 y to <10 y, 2mg):medium dose (10 y to <18 y, 2mg; 2 y to <10 y, 1mg):low dose (10 y to <18 y, 1mg; 2 y to <10 y, 0.5mg):placebo(1:1:1:1) | Wk 16 | vIGA 0/1:- High-dose baricitinib: 41.7% (P<.001)- Medium-dose baricitinib: 25.8% (not significant)- Low-dose baricitinib: 18.2% (not significant)- Placebo: 16.4%EASI75:- High-dose baricitinib: 52.5% (P<.01)- Medium-dose baricitinib: 40% (not significant)- Low-dose baricitinib: 32.2% (not significant)- Placebo: 32% | Acne (2.5%–5%), abdominal pain (2.5%–5%), diarrhea (0.8%–4.2%), upper respiratory tract infection (2.5%–4.2%) | Active smokers |
| Upadacitinib (selective JAK1 inhibitor) | ||||||
| Measure Up 143(NCT03569293) | 847 (12–75 y) | MonotherapyUpadacitinib 15mg:30mg:placebo(1:1:1) | Wk 16 (with follow-up up to wk 52) | vIGA 0/1 (wk 16):- Upadacitinib 15mg: 48.1%c- Upadacitinib 30mg: 62%c- Placebo: 8.4%EASI75 (wk 16):- Upadacitinib 15mg: 69.6%c- Upadacitinib 30mg: 79.9%c- Placebo: 16.3%EASI90 (wk 16):- Upadacitinib 15mg: 53.1%c- Upadacitinib 30mg: 65.8%c- Placebo: 8.1%EASI100 (wk 16):- Upadacitinib 15mg: 16.5%c- Upadacitinib 30mg: 27%c- Placebo: 1.8%Sustained response observed for both doses at wk 52, with numerical superiority for 30mg | Most common AEs (≥5%): Acne (7%–17%), upper respiratory tract infections (9%–13%), nasopharyngitis (8%–12%), headache (5%–7%), CK elevation (6%) | Completed |
| Measure Up 243(NCT03607422) | 836 (12–75 y) | MonotherapyUpadacitinib 15mg:30mg:placebo(1:1:1) | Wk 16 (with follow-up to wk 52) | vIGA 0/1 (wk 16):- Upadacitinib 15mg: 38.8%c- Upadacitinib 30mg: 52%c- Placebo: 4.7%EASI75 (wk 16):- Upadacitinib 15mg: 60.1%c- Upadacitinib 30mg: 72.9%c- Placebo: 13.3%EASI90 (wk 16):- Upadacitinib 15mg: 42.4%c- Upadacitinib 30mg: 58.5%c- Placebo: 5.4%EASI00 (wk 16):- Upadacitinib 15mg: 14.1%c- Upadacitinib 30mg: 18.8%c- Placebo: 0.7%Sustained response observed for both doses at wk 52, with numerical superiority for the 30-mg dose | Most common AEs (≥5%): Upper respiratory tract infection (6%–7%), nasopharyngitis (6%), acne (13%–15%), headache (7%), CK elevation (3%–4%) | Completed |
| AD Up44(NCT03568318) | 901 (12–75 y) | Combined with corticosteroidsUpadacitinib 15mg:30mg:placebo(1:1:1) | Wk 52 | vIGA 0/1 (wk 52):- Upadacitinib 15mg+corticosteroids: 33.5%- Upadacitinib 30mg+corticosteroids: 45.2%- Placebo+corticosteroids: 11.2%EASI75 (wk 52):- Upadacitinib 15mg+corticosteroids: 50.8%- Upadacitinib 30mg+corticosteroids: 69%- Placebo+corticosteroids: 26.3% | Most common AEs (≥5%): Acne (10%–14%), nasopharyngitis (12%–13%), upper respiratory tract infection (7%–8%), CK elevation (4%–6%), oral HSV infection (3%–8%) | Completed |
| Heads Up45(NCT03738397) | 692 (18–75 y) | Head-to-head- Upadacitinib 30mg:dupilumab 300mg every 14 d after initial dose of 600mg(1:1) | 24 wk | EASI75 (wk 16):- Upadacitinib 30mg: 71%c- Dupilumab: 61.1%EASI90 (wk 16):- Upadacitinib 30mg: 60.6%b- Dupilumab: 38.7%EASI75 (wk 24):- Upadacitinib 30mg: 64.2%- Dupilumab: 59.5%EASI90 (wk 24):- Upadacitinib 30mg: 55.6%b- Dupilumab: 47.6%There were large differences in EASI75 responses from wk 2 (43.7% for upadacitinib vs. 17.3% for dupilumab). At wk 24, however, the responses were similar for both drugs. | Most common AEs (≥5%): acne (15.8%), upper respiratory tract infection (6.3%), CK elevation (6.3%) | Completed |
| Abrocitinib (selective JAK1 inhibitor) | ||||||
| JADE MONO-152(NCT03349060) | 387 patients (≥12 y) | MonotherapyAbrocitinib 100mg/24h:abrocitinib 200mg/24h:placebo(2:2:1) | 12 wk | IGA 0/1 (wk 12):- Abrocitinib 100mg: 24%d- Abrocitinib 200mg: 44%b- Placebo: 8%EASI75 (wk 12):- Abrocitinib 100mg: 40%b- Abrocitinib 200mg: 63%b- Placebo: 12%EASI90 (wk 12):- Abrocitinib 100mg: 19%- Abrocitinib 200mg: 39%- Placebo: 5%(CI, no P value)PP-NRS4 (wk 12):- Abrocitinib 100mg: 38%b- Abrocitinib 200mg: 57%b- Placebo: 15%Improvement in itch observed from day 2 of treatment | Most common AEs (≥5%): nausea (9%–20%), nasopharyngitis (12%–15%), headache (8%–10%), upper respiratory tract infection (7%) | Completed |
| JADE MONO-253(NCT03575871) | 391 patients(≥12 y) | MonotherapyAbrocitinib 100mg/24h:abrocitinib 200mg/24h:placebo(2:2:1) | 12 wk | IGA 0/1 (wk 12):- Abrocitinib 100mg: 28.4%b- Abrocitinib 200mg: 38.1%b- Placebo: 9.1%EASI75 (wk 12):- Abrocitinib 100mg: 44.5%b- Abrocitinib 200mg: 61%b- Placebo: 10.4%EASI90 (wk 12):- Abrocitinib 100mg: 23.9%- Abrocitinib 200mg: 37.7%- Placebo: 3.9%(CI, no P value)PP-NRS4 (wk 12):- Abrocitinib 100mg: 45.2%b- Abrocitinib 200mg: 55.3%b- Placebo: 11.5%Improvement in itch observed 24h after treatment initiation | Most common AEs (≥5%): nausea (7.6%–14.2%), nasopharyngitis (7.7%–12.7%), headache (5.7%–7.7%), upper respiratory tract infection (3.2%–8.9%), acne (1.3%–5.2%) | Completed |
| JADE COMPARE55(NCT03720470) | 838 patients(≥18 y) | Combined with corticosteroidsAbrocitinib 200mg/24h:abrocitinib 100mg/24h:dupilumab 300mg every 14 d after initial dose of 600mg:placebo(2:2:2:1) | 16 wk | IGA 0/1 (wk 12 vs. placebo):- Abrocitinib 100mg+corticosteroids: 36.6%b- Abrocitinib 200mg+corticosteroids: 48.4%b- Dupilumab+corticosteroids: 36.5%- Placebo+corticosteroids: 14%EASI75 (wk 12 vs. placebo):- Abrocitinib 100mg+corticosteroids: 58.7%b- Abrocitinib 200mg+corticosteroids: 70.3%b- Dupilumab+corticosteroids: 58.1%- Placebo+corticosteroids: 27.1%Similar efficacy in different regions of the body (head and neck, trunk, upper and lower extremities)The 200- but not the 100-mg dose of abrocitinib was superior to dupilumab in improving itch at wk 2. | Most common AEs (≥5%): Nausea (4.2%–11.1%), nasopharyngitis (6.6%–9.2%), headache (4.2%–6.6%), upper respiratory tract infection (4%–5%) | Completed |
| JADE EXTEND56,57(NCT03422822) | 3000 patients(≥12 y)Long-term extension trial | Corticosteroids allowedAbrocitinib 100mg/24h:abrocitinib 200mg/24h(1:1) | 92 wk | Evaluation of clinical AEs and laboratory abnormalitiesIn nonresponders to dupilumab after 6-wk washout period:IGA 0/1 (wk 12):- Abrocitinib 100mg: 35.2%- Abrocitinib 200mg: 47.2%EASI75 (wk 12):- Abrocitinib 100mg: 67.7%- Abrocitinib 200mg: 80% | Most common AEs: nasopharyngitis (6.9%–11%), nausea (0%–8.2%), acne (2.3%–6.8%), and headache (0.8%–6.8%) | Active (final results not yet published)*Results published to date are shown49 |
| JADE DARE59(NCT04345367) | 727 patients(≥18 y) | Combined with corticosteroidsAbrocitinib 200mg/24h:dupilumab 300mg every 14 d after initial dose of 600mg(1:1) | 26 wk | EASI90 (wk 4):- Abrocitinib 200mg+corticosteroids: 28.5%c- Dupilumab+corticosteroids: 14.6%EASI90 (wk 16):- Abrocitinib 200mg+corticosteroids: 54%b- Dupilumab+corticosteroids: 42%EASI90 (wk 26):- Abrocitinib 200mg+corticosteroids: 55%- Dupilumab+corticosteroids: 48%PP-NRS4 (wk 2)- Abrocitinib 200mg+corticosteroids: 48%c- Dupilumab+corticosteroids: 26%PP-NRS4 (wk 16):- Abrocitinib 200mg+corticosteroids: 67%- Dupilumab+corticosteroids: 64%PP-NRS4 (wk 26)- Abrocitinib 200mg+corticosteroids: 68%- Dupilumab+corticosteroids: 63%Despite the numerical superiority observed for abrocitinib, the differences observed for EASI90 and PP-NRS4 in the first few weeks were no longer significant at wk 26. | AEs more common in patients on abrocitinib 200mg (74%) than on dupilumab (65%)Most common AEs (≥5%) for abrocitinib vs. dupilumab: nausea (19% vs. 2%), headache (13% vs. 7%), acne (13% vs. 11%), and conjunctivitis (3% vs. 11%) | Completed |
| JADE REGIMEN61(NCT03627767) | Of 1233 patients from the 12-wk induction period with abrocitinib 200mg/24h, 798 were randomized(≥12 y) | 12-wk induction with abrocitinib 200mg followed by 40-wk maintenance with abrocitinib 100mg/24h or discontinuation:abrocitinib 200mg/24h:placebo(1:1:1) | Wk 52 (12-wk induction+40 wk) | Possibility of flare (after 40-wk maintenance period):- Abrocitinib 200mg: 18.9%- Abrocitinib 100mg: 42.6%- Placebo: 90.9%After rescue treatment (200mg+corticosteroids), of the patients who experienced a flare 36.6%, 58.8%, and 81.6% regained IGA 0/1 and 55%, 74.5%, and 91.8% regained EASI75 in the abrocitinib 200mg, abrocitinib 100mg, and placebo arms, respectively.Proportion of patients who maintained EASI50 and IGA<3 (wk 52):- Abrocitinib 100mg: 57.4%- Abrocitinib 200mg: 8.1%- Placebo: 19.1% | Most common AEs (≥2%): Nasopharyngitis (3.8%–6.8%), upper respiratory tract infection (3%), headache (0.4%–2.6%), acne (1.9%–3%), anemia (0.4%–2.3%), nausea (0.8%–3%), HSV infection (0.8%–2.6), HZV infection (0.8%–3%) | Completed |
| JADE TEEN62(NCT03796676) | 285 patients (12–17 y) | Combined with corticosteroidsAbrocitinib 200mg/24h:Abrocitinib 100mg:placebo(1:1:1) | 12 wk | IGA 0/1 (wk 12):- Abrocitinib 100mg+corticosteroids: 41.6%a- Abrocitinib 200mg+corticosteroids: 46.2%d- Placebo+corticosteroids: 24.5%EASI75 (wk 12):- Abrocitinib 100mg+corticosteroids: 68.5%d- Abrocitinib 200mg+corticosteroids: 72%b- Placebo+corticosteroids: 41.5%PP-NRS4 (wk 2)- Abrocitinib 100mg+corticosteroids: 27.2%a- Abrocitinib 200mg+corticosteroids: 38.6%b- Placebo: 12.6% | Most common AEs (≥3%): Nausea (7.4%–18.1%), vomiting (4.2%–5.3%) upper respiratory tract infection (9.5%–10.6%), headache (5.3%–8.5%), acne (3.2%–5.3%), CK elevation (4.2%–4.3%) | Completed |
| 2. Topical JAK inhibitors | ||||||
|---|---|---|---|---|---|---|
| Ruxolitinib (selective inhibition of JAK1 and JAK2) | ||||||
| TRuE-AD172(NCT03745638) | 631 patients (≥12 y) | Ruxolitinib 0.75% cream/12h:ruxolitinib 1.5% cream/12h:vehicle/12h(2:2:1) | Wk 8 | IGA-TS (wk 8)Ruxolitinib 0.75% cream: 50%Ruxolitinib 1.5% cream: 53.8%Vehicle: 15.1%PP-NRS4 (wk 8)Ruxolitinib 0.75% cream: 40.4%Ruxolitinib 1.5% cream: 52.2%Vehicle: 15.4% | Application-site burning or stinging (<1%); mild transient increase (within normal range) in platelet counts at week 2 | Finished |
| TRuE-AD272(NCT03745651) | 618 patients (≥12 y) | Ruxolitinib 0.75% cream/12h:ruxolitinib 1.5% cream/12h:vehicle/12h(2:2:1) | Wk 8 | IGA-TS (wk 8)Ruxolitinib 0.75% cream: 39%Ruxolitinib 1.5% cream: 51.3%Vehicle: 7.6%PP-NRS4 (wk 8)Ruxolitinib 0.75% cream: 42.7%Ruxolitinib 1.5% cream: 50.7%Vehicle: 16.3% | Application-site burning or stinging (<1%). Mild transient increase (within normal range) in platelet counts at week 2 | Finished |
| Delgocitinib (pan inhibitor of JAK1, JAK2, JAK3, and TYK2) | ||||||
| JapicCTI-17355469 | Patients (≥16 y) | Delgocitinib 0.5% ointment/12h:vehicle2:1 | Wk 4 | Change in mEASI (wk 4)Delgocitinib 0.5% ointment: −44.3%Vehicle: +1.7%mEASI50 (wk 4)Delgocitinib 0.5% ointment: 51.9%Vehicle: 11.5%Of the patients who continued to receive delgocitinib in the extension study, 69.3% achieved mEASI50 at wk 28. | Kaposi varicelliform eruption, acne, paronychia | Completed |
| JapicCTI-18406470 | 137 patients(2–15 y) | Delgocitinib 0.25% ointment/12h:vehicle2:1 | Wk 4 | Change in mEASI (wk 4)Delgocitinib 0.5% ointment: −39.3%Vehicle: +10.9%mEASI50 (wk 4)Delgocitinib 0.5% ointment: 50.7%Vehicle: 17.6%Of the patients who continued to receive delgocitinib in the extension study, 73.6% achieved mEASI50 at wk 56. | Application-site folliculitis, application-site acne, molluscum contagiosum, viral warts, impetigo | Completed |
Abbreviations, AEs, adverse events; CK, creatine kinase; EASI, Eczema Area and Severity Index; HSV, herpes simplex virus; HZV, herpes zoster virus; mEASI, modified EASI (head/neck region excluded); IGA, Investigator's Global Assessment; IGA-TS, IGA-treatment success (defined as an IGA score of 0 (clear skin [erythema]) or 1 (almost clear skin [hardly any erythema or induration/papulation); JAK, Janus kinase; PP-NRS4, improvement of ≥4 points from baseline in Peak Pruritus Numerical Rating Scale; vIGA, validated IGA.
Baricitinib, which was approved by the European Medicines Agency (EMA) in September 2020 for the treatment of moderate to severe AD in adults, is a first-generation JAK inhibitor that selectively and reversibly blocks JAK1 and JAK2; it has lower affinity for JAK3 and TYK2. It inhibits the signal transduction process of TH2 cytokines, such as IL-4, IL-5, and IL-13, and other cytokines such as IL-22 and IL-31.21
The efficacy of baricitinib combined with topical corticosteroids was first demonstrated in a phase 2 randomized controlled trial (RCT).21 The subsequent phase 3 development program (BREEZE-AD) comprised 7 RCTs, all involving adults.21–27 The replicate trials BREEZE-AD1 and BREEZE-AD2 studied the efficacy of baricitinib monotherapy at daily doses of 1, 2, and 4mg vs. placebo for 16 weeks in 1239 patients. The primary endpoint was an Investigator's Global Assessment (IGA) response of 0/1, which was equivalent to complete or almost complete clearance, with an improvement of 2 or more grades from baseline. Once-daily baricitinib 2mg and 4mg resulted in a higher IGA 0/1 rate than placebo (Table 2). Almost 1 in 4 patients on baricitinib 4mg showed a 75% improvement on the Eczema Area and Severity Index (EASI75) at week 16 of treatment. Treatment was also associated with a rapid improvement in itch, which was significantly greater in the 4-mg group compared with placebo after just 1 week of treatment. Improvements in number of night-time awakenings, skin pain, and quality of life measures were significant for 4mg and 2mg vs. placebo at week 1.27 BREEZE-AD4 studied patients who had not responded to or were intolerant of cyclosporine.25 Finally, the BREEZE-AD7 study analyzed the efficacy of once-daily baricitinib 4mg and 2mg in combination with topical corticosteroids. In brief, 48% of patients on 4mg and 43% of those on 2mg showed an EASI75 response at week 16 compared with 23% on placebo (the difference was nonsignificant for the 2-mg dose). Patients on both 4mg and 2mg reported a significant improvement in itch 24h after the first dose. Long-term results from the BREEZE-AD3 trial were recently published and showing sustained response after 104 weeks of treatment. In BREEZE-AD3, patients who had achieved an IGA 0/1 or IGA 2 (partial) response to once-daily baricitinib 4mg at week 52 were randomized to receive the same dose, 2mg, or placebo. Of the patients still on 4mg at week 104, 47.6% maintained IGA 0/1 (vs. 51.2% at week 52) and 73.8% maintained EASI75 (vs. 82.1% at week 52). Most patients whose dose was lowered to once-daily 2mg maintained their response, although the rates of IGA 0/1 and EASI75 responses were somewhat lower (35.7% and 58.3%, respectively).28
One study of molecular changes following treatment with baricitinib in 124 patients with AD observed a gradual reduction in serum levels of IL-19, a marker of keratinocyte proliferation.29 Another study of 14 Japanese patients found a positive association between AD improvement and a reduction in IL-22 levels after 4 weeks of treatment with baricitinib 4mg.30
Most adverse events (AEs) observed during the clinical development program for baricitinib in AD were mild or moderate, and included asymptomatic creatine kinase (CK) elevations, nasopharyngitis, headache, and diarrhea. Unlike with other JAK inhibitors, patients treated with baricitinib may experience a slight increase in platelet counts, but these have not been linked to AEs. Eczema herpeticum is the most common serious infection reported for baricitinib, with an incidence of 1.4 cases per 100 patient-years in the 2636 patients exposed. The incidence of herpes simplex virus (HSV) infection at 16 weeks was higher for 4mg (6.1%) than for either 2mg (3.6%) or placebo (2.7%), with an overall incidence of 6.7 cases per 100 patient-years in all patients treated with baricitinib. The incidence rate of major adverse cardiovascular events (MACE) was 0.15 cases per 100 patient-years, and there were 3 cases of pulmonary thromboembolism (incidence rate, 0.06). None of these events were considered to be attributable to baricitinib, since the incidence was similar to that observed in the general population and the patients had associated risk factors.31 A recent study that stratified patients according to individual risk factors compared the safety of baricitinib in patients with rheumatoid arthritis, alopecia areata, and AD. The incidence of AEs of interest (e.g., MACE, thromboembolic disease, and malignancies) in patients with AD and alopecia areata was similar to that reported in the literature, regardless of other risk factors such as older age (≥65 years). Treatment decisions nonetheless should be tailored to individual risk and disease burden.32
Overall, baricitinib 4mg and baricitinib 2mg both have acceptable safety profiles but the 4-mg dose is associated greater treatment responses and faster onset of action compared with placebo. The recommended dose33 thus is 4mg once daily. The 2-mg dose administered once a day is recommended for special circumstances (Table 3); doses can also be optimized in subgroups of treated patients.34
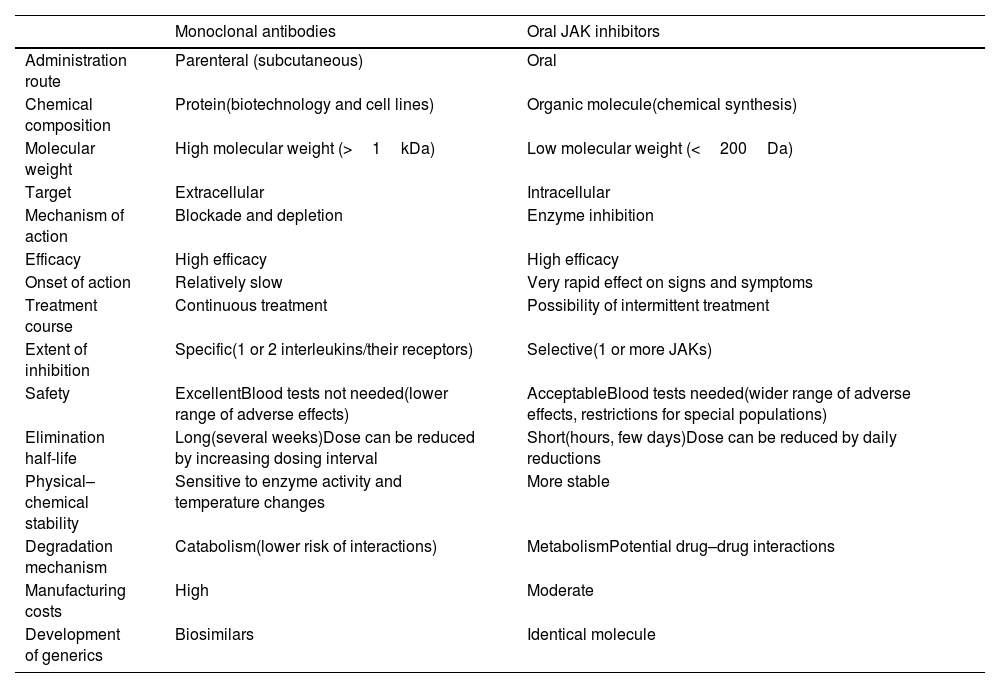
General Characteristics of Monoclonal Antibodies and JAK Inhibitors.
| Monoclonal antibodies | Oral JAK inhibitors | |
|---|---|---|
| Administration route | Parenteral (subcutaneous) | Oral |
| Chemical composition | Protein(biotechnology and cell lines) | Organic molecule(chemical synthesis) |
| Molecular weight | High molecular weight (>1kDa) | Low molecular weight (<200Da) |
| Target | Extracellular | Intracellular |
| Mechanism of action | Blockade and depletion | Enzyme inhibition |
| Efficacy | High efficacy | High efficacy |
| Onset of action | Relatively slow | Very rapid effect on signs and symptoms |
| Treatment course | Continuous treatment | Possibility of intermittent treatment |
| Extent of inhibition | Specific(1 or 2 interleukins/their receptors) | Selective(1 or more JAKs) |
| Safety | ExcellentBlood tests not needed(lower range of adverse effects) | AcceptableBlood tests needed(wider range of adverse effects, restrictions for special populations) |
| Elimination half-life | Long(several weeks)Dose can be reduced by increasing dosing interval | Short(hours, few days)Dose can be reduced by daily reductions |
| Physical–chemical stability | Sensitive to enzyme activity and temperature changes | More stable |
| Degradation mechanism | Catabolism(lower risk of interactions) | MetabolismPotential drug–drug interactions |
| Manufacturing costs | High | Moderate |
| Development of generics | Biosimilars | Identical molecule |
Abbreviation: JAK, Janus kinase.
A phase 3 RCT (NCT03952559) is currently investigating baricitinib in children and adolescents aged 2 to 17 years. Preliminary results show greater benefits for the higher doses (4mg in patients aged 10 to 17 years and 2 mg in those aged 2 to 9 years), with 41.7% of patients achieving IGA 0/1 and 52.5% achieving EASI75 at week 16.35
The first real-world data on the use of baricitinib in adults were recently published, but they are still limited in terms of number of patients, treatment duration, and assessment of dose optimization.36–39 In a Japanese series of 14 patients treated with baricitinib, 64% showed an EASI75 response and 36% an EASI90 response at week 12. According to another Japanese study of 36 patients, baricitinib was more likely to induce a response in the lower extremities than in the head and neck region by week 12.40 In a German series of 12 patients treated with baricitinib, 90% achieved EASI75 at week 12.38 Data on 51 patients from the Dutch Bioday registry treated with baricitinib showed a 22% likelihood of an IGA 0/1 response; this rate is similar to rates described in clinical trials. Treatment was discontinued in one-third of patients due to lack of effectiveness.39 Baricitinib has also been used in patients with AD and concomitant alopecia areata (for which this treatment is also indicated).41
UpadacitinibUpadacitinib is a selective JAK1 inhibitor. It was approved by the EMA in August 2021 for the treatment of moderate to severe AD in patients aged 12 years or older.42 Its use in Spain was authorized in April 2022.
Two replicate phase 3 RCTs (Measure Up 1 and Measure Up 2) compared the efficacy of once-daily upadacitinib 15mg and 30mg as monotherapy vs. placebo in patients with moderate to severe AD aged 12–75 years.43 The primary endpoints for both doses (vIGA 0/1 and EASI75) were reached at week 16, both in Measure Up 1 (validated IGA [vIGA] 0/1: upadacitinib 15mg/48.1%, upadacitinib 30mg/62%, placebo 8.4% [P<0.0001] and EASI75: upadacitinib 15mg/69.6%, upadacitinib 30mg/79.9%, placebo/16.3% [P<0.0001]) and Measure Up 2 (vIGA 0/1: upadacitinib 15mg/38.8%, upadacitinib 30mg/52%, placebo/4.7% [P<0.0001] and EASI75: upadacitinib 15mg/60.1%, upadacitinib 30mg/72.9%, placebo/13.3% [P<.0001]). Secondary endpoints were also achieved with both doses. The incidence of serious AEs and events leading to drug discontinuation were similar in the 3 groups. The incidence of herpes zoster virus (HZV) infection was higher in patients treated with upadacitinib 15 or 30mg (20 cases [1.8%] in 1124 patients) than in those treated with placebo (2 cases [<1%] in 559 patients). Treatment was not discontinued in any of the cases.43 Another phase 3 RCT (AD Up) evaluated the efficacy and safety of upadacitinib combined with topical corticosteroids up to week 52. Again, the primary endpoints (vIGA 0/1 and EASI75) were achieved for both doses, with favorable results observed for both efficacy and onset of action. Significant differences were observed from week 2 of treatment, with a sustained response at week 52. No new safety issues emerged during this follow-up period.44,45
Finally, the Heads Up trial compared once-daily upadacitinib 30mg and dupilumab in adults over 24 weeks.46 By week 16, 71% of those treated with upadacitinib 30mg had achieved EASI75 compared with 61.1% in the dupilumab group (P=.006). Upadacitinib was also associated with more rapid onset of action, with 43.7% of patients achieving EASI75 by week 2 in the upadacitinib group compared with 17.4% in the dupilumab group. At week 16, a higher proportion of patients on upadacitinib achieved EASI90 (60.6% vs. 38.7% for dupilumab) and EASI100 (23.2% vs. 7.6% for dupilumab) (P<001). The response at week 24 was similar for both drugs. Eczema herpeticum, HZV infection, and laboratory abnormalities were more common in the upadacitinib group, while conjunctivitis and injection-site reactions were more common in the dupilumab group. One death due to influenza-associated bronchopneumonia occurred in the upadacitinib group.
A growing number of publications are reporting on the real-world use of upadacitinib in both adults and pediatric patients aged 12 years or older with AD. The response rates are similar to those described in the RCTs, and 1 notable potential AE is acne.47–49 Resolution of dupilumab-induced conjunctivitis with good AD control has been described after a switch to upadacitinib.50
AbrocitinibAbrocitinib is a selective JAK1 inhibitor approved by the EMA in December 2021 for the treatment of moderate to severe AD in adults.51 Its efficacy as monotherapy (once-daily 100mg or 200mg) vs. placebo at 12 weeks was evaluated in 2 replicate RCTs (JADE MONO-152 and JADE MONO-253) involving adults and adolescents. EASI75 and IGA 0/1 responses were superior for both doses compared with placebo (Table 2). AEs were more common in patients on abrocitinib, but there were no differences with placebo for serious events. The most common AEs in the abrocitinib groups were nausea, headache, nasopharyngitis,52,53 and acne (which was dose dependent).53
The 16-week JADE COMPARE trial evaluated the efficacy and safety of abrocitinib 100mg and 200mg vs. standard-dose dupilumab and placebo. All patients received concomitant topical corticosteroids. Overall, 70.3% of patients on abrocitinib 200mg and 58.7% of those on abrocitinib 100mg achieved EASI75; the corresponding rates in the other groups were 58.1% for dupilumab and 27.1% for placebo. The only significant difference observed for secondary endpoints between abrocitinib and dupilumab was an improvement in itch at week 2 in patients on abrocitinib 200mg.54,55 The patients from JADE MONO-1, JADE MONO-2, and JADE COMPARE were given the option of continuing in a long-term extension trial (JADE EXTEND) where they received once-daily abrocitinib 100mg or 200mg with the possibility of using concomitant topical corticosteroids for 92 weeks. The final results of this trial are pending publication.56 According to the preliminary results, patients from the JADE COMPARE trial who had received dupilumab up to week 14 were switched to oral placebo for 6 weeks (week 14–20) before entering JADE EXTEND, where they were rerandomized to once-daily abrocitinib 200mg or 100mg. In the subgroup of patients who had not responded to dupilumab, EASI75 at week 12 was reached by 80% of those treated with abrocitinib 200mg and 67.7% of those treated with 100mg.57 Nonetheless, it should be considered that some residual effects of dupilumab might have remained, despite the 6-week placebo washout (week 14–20).58
The JADE DARE trial compared abrocitinib 200mg vs. dupilumab, both in combination with topical corticosteroids.59 Primary endpoints were EASI90 and an improvement of 4 points or higher on the Peak Pruritus Numerical Rating Scale (PP-NRS4). Although a higher proportion of patients on abrocitinib 200mg reached EASI90 (28.5% vs. 14.6% for dupilumab) and PP-NRS4 (48% vs. 26%) by week 2, the differences between the groups decreased with time, similarly to the case of upadacitinib vs. dupilumab in the Heads Up trial.60
The JADE REGIMEN trial, which lasted 52 weeks, started with a 12-week open-label induction period with once-daily abrocitinib 200mg administered to patients aged 12 years or older.61 Patients who achieved EASI75 and IGA 0/1 during this period (64.7%) were randomized to receive abrocitinib 100mg, abrocitinib 200mg, or placebo for 40 weeks. The proportion of patients who maintained EASI50 and still had an IGA <3 was higher for abrocitinib 200mg (81.1%) than for either abrocitinib 100mg (57.4%) or placebo (19.1%). In brief, both doses were associated with a more sustained response than placebo. Rescue treatment with once-daily abrocitinib 200mg plus topical corticosteroids allowed most nonresponders to regain IGA 0/1 and EASI75. The JADE TEEN trial reported similar results for the efficacy and safety of abrocitinib combined with topical corticosteroids in adolescents aged 12 to 17 years (Table 2).62,63
A safety profile analysis of abrocitinib using data from 3582 patients treated within the JADE clinical program (exposure, 4313.4 person-years) reported a higher incidence of severe AEs in patients aged 65 years or older. The most common serious AEs were serious infections, with an incidence of 2.46 (1.79–3.31) cases for abrocitinib 200mg and 2.43 (1.66–3.44) for abrocitinib 100 mg. The most common infections in this category were HZV infection, herpes simplex virus (HSV) infection, and pneumonia.64 Overall, once-daily abrocitinib 200mg is more effective in the treatment of AD, although it is associated with a higher incidence of mild and moderate adverse effects than once-daily abrocitinib 100mg.65
Other Oral JAK InhibitorsGusacitinib (ASN002)Gusacitinib is the first dual oral JAK/SYK inhibitor. In addition to inhibiting all 4 JAKs, it inhibits spleen tyrosine kinase (SYK), which regulates B-cell and dendritic-cell differentiation and TH17/IL-17 signaling. SYK inhibition can be used to attenuate the TH17 pathway and improve the terminal differentiation of keratinocytes, which could provide additional benefits to those offered by JAK inhibition (attenuation of the TH1, TH2, and TH22 pathways) in certain endotypes.66 A phase 1b RCT compared gusacitinib at daily doses of 20, 40 and 80mg vs. placebo for 4 weeks. EASI50 responses were more common in patients on gusacitinib 40mg (100%) and 80mg (83%) than in those on placebo (22%).67 A phase 2b RCT (NCT03531957) found no differences in the absolute reduction of EASI compared with placebo and the extension study was terminated.66 There have been no new studies of gusacitinib in AD. Its use in chronic hand eczema, however, is still being evaluated.66
TofacitinibTofacitinib is a JAK1 and JAK3 inhibitor. Oral tofacitinib at 5mg/12h and 5mg/24h were studied in a proof-of-concept study involving 6 adults with AD. The treatment was associated with a 66.6% reduction from baseline in the SCORAD (SCORing Atopic Dermatitis) index during 8 to 29 weeks of treatment. It does not currently seem likely that tofacitinib will achieve approval for use in AD.68
Topical JAK InhibitorsTopical therapy is one of the mainstay treatments for AD. A number of topical JAK inhibitors are currently being investigated; these could provide local benefits without the risk of systemic AEs associated with orally administered inhibitors.
DelgocitinibDelgocitinib is a first-generation pan-JAK inhibitor (JAK1, JAK2, JAK3, and TYK2). Its efficacy and safety was evaluated in a vehicle-controlled RCT, whose results have been published. The trial studied 158 patients aged 16 years or older: 106 applied delgocitinib and 52 applied the vehicle only twice a day for 4 weeks. The primary endpoint was a reduction in modified EASI (mEASI), which was calculated by excluding the head and neck region score from the EASI. The change in mEASI was significantly greater for delgocitinib than for the vehicle (−44.3 vs. +1.7%, respectively, P<.001). In addition, 51.9% of patients in the delgocitinib group vs. 11% of those in the placebo group achieved a reduction of at least 50% from baseline (mEASI50). Improvement in itch was observed after the first day of treatment. The RCT continued with a 24-week open-label extension period in which 95% of patients reached mEASI50 and 49% mEASI75; the safety profile was acceptable. There were 3 cases of Kaposi varicelliform eruption associated with delgocitinib. No lymphopenias were reported.69 Similar results, with no new safety alerts, were described for a phase 3 RCT in pediatric patients (≥2 and ≤15 years) treated with delgocitinib 0.25% and 0.5% (Table 2).70 The limitation of the trial was that it was only conducted in Japanese patients. Delgocitinib 0.5% ointment was approved for the treatment of AD in Japan in January 2020.71 Outside Japan, the efficacy and safety of delgocitinib at different concentrations (1, 3, 8 and 20mg/g) is being studied in adults (NCT03725722) and pediatric patients aged 2 years and older (NCT03826901) with AD affecting a body surface area of less than 50%. The potential benefits of delgocitinib in the treatment of chronic hand eczema are also being investigated (NCT04871711, NCT04872101).
RuxolitinibRuxolitinib is a selective JAK1 and JAK2 inhibitor. Two phase 3 RCTs (TRuE-AD1 and TRuE-AD2) evaluated the efficacy of ruxolitinib 0.75% and 1.5% applied every 12hours for 8 weeks in 1249 patients with AD aged 12 years or older (Table 2). About 50% of patients treated with ruxolitinib achieved the primary endpoint (IGA-treatment success [IGA-TS] 0/1), and patients in the 1.5% group reported a significant reduction in itch at 12hours after the first application. 71 Treatment success was maintained at week 52, with 74.1% to 77.8% of patients showing an IGA-TS 0/1.72 Application of ruxolitinib to a body surface area of up to 20% was not associated with significant plasma concentrations of the drug in pharmacokinetic studies.71 In October 2021, topical ruxolitinib was approved by the US Food and Drug Administration for short-term or intermittent treatment of AD in patients aged 12 years or older, making it the first topical JAK inhibitor to be approved for this indication.73 Although ruxolitinib was evaluated as monotherapy, it could be used in combination with other topical or systemic treatments.
BrepocitinibBrepocitinib is a topical JAK1 and TYK2 inhibitor under development for the treatment of AD. The results of the phase 2b trial evaluating the efficacy and safety of brepocitinib at different concentrations vs. vehicle were recently published. The concentrations tested were 0.1% every 24hours, 0.3% every 24hours or 12hours, and 1% every 24hours or 12hours; the vehicle was applied every 24hours or 12hours. At week 6, patients in the brepocitinib 1% groups achieved an EASI change of −70.1% (−82.1% to −58%) for once-daily application and of −75% (−83.8% to 66.2%) for twice-daily application. In the vehicle-only group, the change was −44.4% [−57.3 to −31.6%] for once-daily application and −47.6% [−57.5% to −37.7%]) for twice-daily application.74
TofacitinibThe results of a phase 2a randomized trial of topical tofacitinib 2% were published in 2016. Twice-daily application for 4 weeks was associated with a mean EASI reduction of 81.7% vs. 29.9% for placebo.71 No other studies investigating its use in AD have been registered.
Cerdulatinib (DMVT-502)Cerdulatinib is a dual JAK/SYK inhibitor. The only study to analyze its use in AD was a phase 1b trial that studied twice-daily application in a 0.37% gel formulation for 14 days. The results showed significant reductions in EASI.71 No new clinical trials have been registered since for its use in AD.
Clinical Use ConsiderationsChoice of TreatmentA number of recent systematic reviews and meta-analyses have analyzed the use of biologics and JAK inhibitors in AD. Their findings show high efficacy for both upadacitinib and abrocitinib, with the highest doses outperforming dupilumab. Overall, they showed a favorable safety profile for JAK inhibitors in this setting, but there are potential safety issues to consider, and AEs are frequently dose dependent.75,76 A number of factors should thus be taken into account when deciding on individual treatment.
Although these novel treatments for AD each have their differences, there are 2 basic strategies based on the 2 major groups of novel systemic therapies: monoclonal antibodies or biologic – which offer greater specificity – and JAK inhibitors – which are nonspecific option but offer greater or lesser selectivity based on the inhibition of 1 or more JAKS (Table 3).
Faster onset of action is one of the main benefits of JAK inhibitors, which have shown rapid and marked improvements in signs and symptoms. This is potentially of interest for patients who require fast response, such as those with a high disease burden or erythrodermic AD, where rapid action can reduce the risk of morbidity and mortality. Because of the drugs’ rapid onset of action and short elimination half-life, treatment cycles can also be considered, particularly in patients with intermittent or seasonal AD. Another advantage of this short elimination half-life is that it enables rapid reversal of certain adverse effects on discontinuation of the drug. Likewise, because JAK inhibitors induce a broader inhibition of cytokines than monoclonal antibodies, they could theoretically be useful for forms of AD with significant participation by non-TH2 pathways, such as psoriasiform atopic eczema. Their use should also be contemplated in patients who could benefit from the simultaneous treatment of concomitant diseases, in particular non-TH2-mediated inflammatory or autoimmune diseases. Baricitinib, for example, is approved for use in alopecia areata, and upadacitinib has been authorized for arthritis and inflammatory bowel disease. Its use in vitiligo and hidradenitis suppurativa is being investigated. JAK inhibitors may also be beneficial in patients with severe basal ocular surface involvement, which can potentially be worsened by the use of biologics in AD. A similar case could hold for patients treated with monoclonal antibodies who develop persistent or refractory adverse effects, mainly involving the eyes or skin.
The biologic dupilumab has advantages in that it is indicated for other TH2-cell-driven diseases such as asthma. The European expert consensus statement on the systemic treatment of AD in special populations recommends prioritizing biologics over JAK inhibitors in patients older than 65 years, patients with a history of cancer, and patients with frequent or chronic infections such as hepatitis B virus infection.77 Real-world data have shown a favorable safety profile for dupilumab in transplant recipients and patients with severe chronic kidney disease.78 There is also evidence suggesting that it may be safely used in pregnant or breastfeeding women.79
Choice of treatment will ultimately be determined by guidelines or regional- or hospital-based protocols, patient preferences (e.g., oral vs. subcutaneous administration), and medical judgment in relation to individual patient profiles, risks and comorbidities, and the likelihood of response according to the latest evidence. Table 3 summarizes the main characteristics and potential advantages and disadvantages of monoclonal antibodies and JAK inhibitors.
Safety of JAK InhibitorsOverall, clinical trials, which have included an extensive clinical development program for baricitinib, upadacitinib, and abrocitinib, have demonstrated an acceptable safety profile for oral JAK inhibitors in AD. Most emergent AEs were mild and transient, and no significant differences were observed between these drugs and placebo during the trial periods. Headache and nausea may occur in around 10% of patients, with somewhat higher rates for baricitinib and abrocitinib; other events include acne and/or folliculitis, with a possibly higher incidence with upadacitinib.35 Emerging real-world data will help better characterize these findings. One notable issue related to the inhibition of IFN responses is increased susceptibility to viral infections, with reports of increased reactivation of HSV – mostly oral – and HZV infections. There have been no reports of reactivation of tuberculosis or opportunistic infections. JAK inhibitors, however, have not been studied in combination with other immunosuppressants such as cyclosporine or methotrexate. Their concomitant use is not recommended in patients with AD as it could increase the risk of immunosuppression and serious infections.80
Notable class adverse effects associated with JAK inhibitors include CK elevations, but most of these have been grade 1 and have not been linked to clinical repercussions. Increases in low-density lipoproteins (LDL) and high-density lipoproteins (HDL) have also been detected, but they have not affected the LDL:HDL ratio. Long-term data are needed to investigate a possible association with cardiovascular AEs. JAK inhibitors may be associated with neutropenia, lymphopenia, or anemia, but these blood alterations have been seldom reported in patients with AD and most of them have been grade 1. Thrombocytosis may occur in patients treated with baricitinib, and there have been reports of thrombocytopenia in patients on abrocitinib. In both cases, platelet levels would appear to reach their lowest or highest point at week 4, but then return to baseline levels during treatment.18,81–83
One of the main safety alerts associated with JAK inhibitors is the risk of thromboembolic disease. Very few cases of deep vein thrombosis or pulmonary thromboembolism have been reported in published trials, and most of them occurred in patients with pre-existing risk factors, such as older age, smoking, or prolonged immobilization. Due to the low overall incidence of these events in the general population, it is difficult to determine the strength of association with JAK inhibitors. The recommendation is thus to consider individual risk factors whenever selecting a treatment. Based on current evidence from trials, JAK inhibitors are not associated with an increased risk of tumors relative to the general population.18,81–83
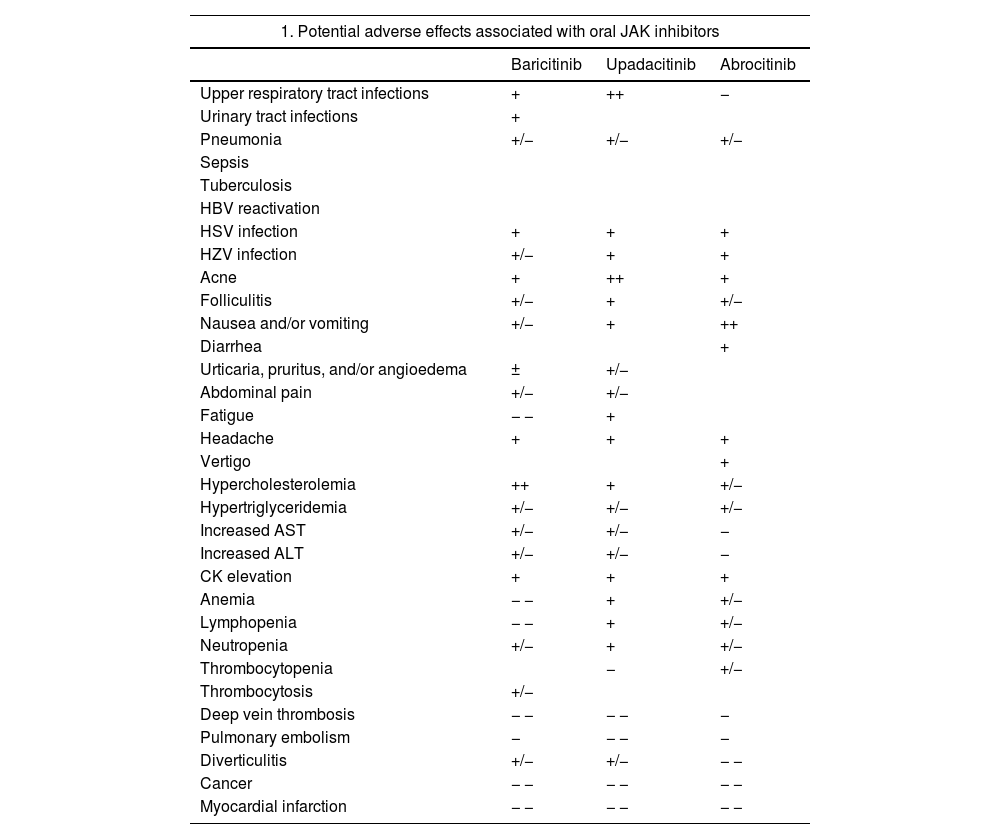
Despite the low incidence of serious AEs described for JAK inhibitors in patients with AD, on January 23, 2023, the EMA published recommendations to reduce associated risks in patients with chronic inflammatory diseases. The guidelines recommend only using JAK inhibitors in the following patients; patients for whom no other suitable treatments are available, patients aged 65 years or older, patients with an increased risk of serious cardiovascular disease such as stroke and myocardial infarction, smokers (or ex-smokers with a history of high exposure), and patients at risk of cancer.84 Potential common and uncommon AEs associated with oral JAK inhibitors are listed in Table 4 together with EMA treatment recommendations.18,81–84
Safety of JAK Inhibitors.
| 1. Potential adverse effects associated with oral JAK inhibitors | |||
|---|---|---|---|
| Baricitinib | Upadacitinib | Abrocitinib | |
| Upper respiratory tract infections | + | ++ | − |
| Urinary tract infections | + | ||
| Pneumonia | +/− | +/− | +/− |
| Sepsis | |||
| Tuberculosis | |||
| HBV reactivation | |||
| HSV infection | + | + | + |
| HZV infection | +/− | + | + |
| Acne | + | ++ | + |
| Folliculitis | +/− | + | +/− |
| Nausea and/or vomiting | +/− | + | ++ |
| Diarrhea | + | ||
| Urticaria, pruritus, and/or angioedema | ± | +/− | |
| Abdominal pain | +/− | +/− | |
| Fatigue | − − | + | |
| Headache | + | + | + |
| Vertigo | + | ||
| Hypercholesterolemia | ++ | + | +/− |
| Hypertriglyceridemia | +/− | +/− | +/− |
| Increased AST | +/− | +/− | − |
| Increased ALT | +/− | +/− | − |
| CK elevation | + | + | + |
| Anemia | − − | + | +/− |
| Lymphopenia | − − | + | +/− |
| Neutropenia | +/− | + | +/− |
| Thrombocytopenia | − | +/− | |
| Thrombocytosis | +/− | ||
| Deep vein thrombosis | − − | − − | − |
| Pulmonary embolism | − | − − | − |
| Diverticulitis | +/− | +/− | − − |
| Cancer | − − | − − | − − |
| Myocardial infarction | − − | − − | − − |
| 2. EMA recommendations to minimize risk of serious adverse effects with JAK inhibitors. |
|---|
| Systemic JAK inhibitors should only be used in patients with 1 or more of the following factors when no other suitable treatments are available:Age ≥65 y• Present or past history of smoking• Additional cardiovascular risk factors• Risk factors for cancers |
| JAK inhibitors should be used with caution in patients with risk factors for thromboembolic disease:• History of thromboembolic disease• Major surgery• Prolonged immobilization• Combined hormonal contraceptives• Hormone replacement therapy• Hereditary coagulation disorder• History of myocardial infarction• Heart failure• Additional risk factors: obesity, diabetes mellitus, hypertension |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HSV, herpes simplex virus; HZV, herpes zoster virus; JAK, Janus kinase.
Note: The adverse effects listed are based on the respective summaries of product characteristics. Note that the information is reported in the aggregate, including the different doses and potential populations studied for each of the drugs. The frequencies specified will vary according to dose, the disease being treated, and individual patient factors, such as age. Estimated frequencies: very common ++ (≥1/10), common + (≥1/100 to <1/10), uncommon +/− (≥1/1000 to <1/100), rare − (≥1/10000 to <1/1000), very rare − − (<1/10000). Not reported.
Factors that should be assessed before and during treatment are summarized in Table 5. These are based on the summaries of product characteristics of oral JAK inhibitors approved for use in AD and reports of their use in other indications, mainly rheumatic arthritis.80,85-87
Practical Considerations of New JAK Inhibitors.
| Pretreatment evaluation |
| 1. History taking and physical examination• Estimation of overall risk of thromboembolic disease (known personal or family history, obesity, smoking, oral contraceptive use, prolonged immobilization, age >45 years, major surgical interventions (e.g., neurosurgery or urological, gynecological, or orthopedic surgery), hereditary thrombophilia (factor V Leiden, prothrombin mutation 20210), acquired thrombophilia (e.g., antiphospholipid syndrome), cancer.• Estimation of overall cardiovascular risk (personal cardiovascular risk factors, concomitant use of cyclooxygenase-2 inhibitors, prednisone >7.5mg/24h, oral contraceptives)• Skin examination (baseline evaluation for nonmelanoma skin cancer)2. Baseline blood tests (complete blood count and biochemistry):• Complete blood count (including differential white blood cell count)• Liver function (in particular, transaminases)• Kidney function• Lipid levels• Routine creatine kinase not recommended3. Infection risk assessment:• HBV serology (anti-HBs, anti-HBc, and anti-HBsAg)∘ Use not recommended in patients with chronic HBV infection∘ In patients with prior exposure (anti-HBc+) but no evidence of active replication (HBsAg−), test for HBV-DNA∘ In patients with anti-HBc+ and HBsAG− but without HBV-DNA, JAK inhibitor therapy can be started following a risk-benefit assessment and with close monitoring in coordination with the hepatology department.• HCV serology:∘ Test for HCV RNA. If positive, complete full HCV treatment before starting JAK inhibitor.HIV serology recommended• TB screening recommended. The risk of TB reactivation with JAK inhibitors could be similar to that observed for TNF inhibitors.• Chest radiograph• Tuberculin test and/or interferon gamma release assay• If treatment is required, do not start JAK inhibitor until 1 to 2 months after TB treatment.• Coadministration with rifampicin (a CYP 3A4 inducer) could diminish response to upadacitinib.• Varicella zoster serology in patients without a previous history of immunization• Other serologies: measles, rubella, and parotiditis (immunization status)4. Evaluation of immunization status• Follow national and regional vaccination guidelines.• Check history of chickenpox and HZV infection and previous vaccination:∘ Risk factors for HZV reactivation include age >50 years, female sex, concomitant use of corticosteroids, infection, and hospitalization.∘ Prior vaccination with the recombinant herpes zoster vaccine (Shingrix, 2 doses administered 2 months apart) is recommended for people aged >18 years. The recombinant vaccine is not contraindicated during treatment with JAK inhibitors, but it could affect their immunogenicity.∘ Live and live attenuated vaccines are contraindicated in patients on JAK inhibition therapy.5. Pregnancy and breastfeeding. JAK inhibitors are contraindicated during pregnancy and breastfeeding. Women on JAK inhibition therapy should use suitable contraceptive methods.• Baricitinib: contraception during and for at least 1 week after treatment• Upadacitinib: contraception during and for at least 4 weeks after treatment• Abrocitinib: contraception during and for at least 4 weeks after discontinuation• Discontinuation of JAK inhibitors prior to conception is not necessary in men. No effects on spermatogenesis have been demonstrated.6. Evaluation of concomitant medication• Drug–drug interactions∘ Baricitinib: OAT3 inhibitors → ↑[baricitinib] (e.g., probenecid; recommended dose, 2mg/24h)• Upadacitinib:• CYP3A4 inhibitors → ↑[upadacitinib] (e.g., ketoconazole, itraconazole, posaconazole, voriconazole, and clarithromycin). Avoid 30mg/24h dose. Consider alternatives to potent CYP3A4 inhibitors if used long term.• CYP3A4 inducers → ↓[upadacitinib] (e.g., rifampicin and phenytoin)• Abrocitinib:• CYP2C19/CYP2C9 inhibitors: ↑[abrocitinib] (e.g., fluvoxamine and fluconazole)• CYP2C19/CYP2C9 inducers: ↓[abrocitinib] (e.g., rifampicin)• Increased risk of immunosuppression when combined with other drugs. The use of JAK inhibitors with cyclosporine or other systemic immunosuppressants is not recommended in AD. |
| Clinical follow-up and blood tests during treatment |
| 1. Assess for individual infection risk (e.g., occupational exposure, travel, comorbidities, concomitant medications, signs or symptoms of infection)2. Blood tests are recommended at 3 months and thereafter every 6 months. An initial evaluation can be contemplated after 4 weeks of treatment in patients with certain risk factors• Complete blood count:∘ Hemoglobin levels (confirmed in 2 consecutive blood tests):• ≤ 8g/dL and/or reduction >2g/dL from baseline: interrupt treatment until levels return to normal. The decision to restart treatment should be considered on a case-by-case basis with assessment of risks and benefits.• >9g/dL: no dose adjustment required∘ Hemoglobin levels (confirmed in 2 consecutive blood tests):• <500mm3: stop treatment• 500–1000mm3: reduce dose or stop treatment until levels return to >1000mm3• >1000mm3: no dose adjustment required∘ Absolute lymphocyte counts (in 2 consecutive blood tests):• <500mm3: stop treatment• 500–750mm3: reduce dose or stop treatment until levels return to >750mm3• >750mm3: no dose adjustment required• Evaluate liver function (see adjustments for specific JAK inhibitor)• Evaluate kidney function (see adjustments for specific JAK inhibitor)• Monitor lipid levels (recommended at month 3 of treatment). If dyslipidemia is detected, follow standard treatment guidelines. Potential interactions between various statins and JAK inhibitors have been evaluated.• The combination of statins with baricitinib, upadacitinib, or abrocitinib does not appear to have relevant pharmacokinetic effects.• CK levels do not need to be tested unless there is a clinical indication (e.g., myalgia)3. Monitoring of infection risk:• Annual serology for HBV, HCV, and HIV is only indicated for patients at risk for these conditions.• Periodic (e.g., annual) screening for latent TB should be considered in risk groups for latent TB (e.g., occupational exposure).• If a patient develops HZV infection during treatment, consider stopping the JAK inhibitor until the episode resolves.4. Evaluation of cancer risk: follow national screening recommendations for age and sex. Skin examination (nonmelanoma skin cancer and melanoma)5. Vaccination: Inactivated vaccines can be administered during JAK inhibition therapy, although their immunogenicity could be decreased. |
| Specific considerations for oral JAK inhibitors. | |||
|---|---|---|---|
| Baricitinib | Upadacitinib | Abrocitinib | |
| Standard dose | ≥18–74 y:4mg/24h≥75 y:2mg/24h | >18–64 y:30mg in patients with a high disease burden or inadequate response to 15mg. Lowest effective dose for maintenance therapy.≥65 y and adolescents >12 y and >30kg: 15mg/24h*Limited data available for >75 y: use with caution | >18–64 y:200mg/24h≥65 y:100mg/24h*Limited data available for >75 y: use with caution |
| Dose adjustment: kidney failure | eGFR >30–60mL/min: 2mg/24heGFR <30mL/min: not recommended | eGFR >30–60mL/min: no dose adjustment requiredeGFR 15–30mL/min: 15mg/24heGFR <15mL/min: not recommended | eGFR >30–60mL/min: 100mg/24heGFR 15–30mL/min: 50–100mg/24heGFR <15mL/min: not recommended |
| Dose adjustment:Liver failure | Child-Pugh A–B: no dose adjustment requiredChild-Pugh C: not recommended | Child-Pugh A–B: no dose adjustment requiredChild-Pugh C: not recommended | Child-Pugh A–B: no dose adjustment requiredChild-Pugh C: not recommended |
JAK inhibitors could change the treatment landscape for AD. They are highly effective, have a rapid onset of action, and simultaneously inhibit several cytokines, a relevant feature considering the highly variable phenotypes and endotypes that characterize AD. Although the general safety profile of JAK inhibitors is adequate, the risk of laboratory abnormalities and serious AEs, while low, warrants careful evaluation of individual risk factors during treatment decisions and follow-up. Real-life data will provide useful insights into efficacy and safety, as will experience with the use of JAK inhibitors in different scenarios, such as continuous long-term treatment and shorter or intermittent strategies.
FundingThis study received no funding.
Conflicts of InterestM. Munera-Campos has received fees for consultancy services, presentations, and other related activities from Abbvie, Leo-Pharma, Janssen, Sanofi, and Galderma. She has also served as a principal or co-investigator on clinical trials sponsored by Lilly, Leo-Pharma, Novartis, Janssen, Sanofi, Pfizer, Abbvie, Almirall, UCB, and Galderma. José Manuel Carrascosa has served as a principal or co-investigator and/or received speaker's fees, and/or served on expert or steering committees for Abbvie, Novartis, Janssen, Lilly, Sandoz, Amgen, Almirall, BMS, Boehringer Ingelheim, Biogen, and UCB.