Radiotherapy for cancer is used increasingly. Because skin cells undergo rapid turnover, the ionizing radiation of radiotherapy has collateral effects that are often expressed in inflammatory reactions. Some of these reactions–radiodermatitis and recall phenomenon, for example–are very familiar to dermatologists. Other, less common radiotherapy-associated skin conditions are often underdiagnosed but must also be recognized.
La radioterapia es una técnica de uso creciente en el campo de la oncología. Debido al alto recambio celular cutáneo, la radiación ionizante afecta colateralmente a la piel y encontramos de forma frecuente dermatosis inflamatorias asociadas a radioterapia. Algunos de estos cuadros, como la radiodermitis o el fenómeno de recall, son bien conocidos por el dermatólogo. Es importante reconocer otros cuadros cutáneos asociados a radioterapia que aparecen de forma menos frecuente y que en muchas ocasiones son infradiagnosticados.
Radiotherapy is used often in oncology: it is prescribed for nearly half of cancer patients as monotherapy or in combination with other treatments. In the skin, where the rate of cell turnover is high, ionizing radiation has collateral effects that make adverse reactions very common. Up to half of patients reportedly develop at least mild radiodermatitis,1 and there is a small percentage of patients who also present more serious skin conditions affecting large surface areas.
We propose a classification system for skin conditions that appear exclusively in the context of radiotherapy and that may or may not be dose dependent (Table 1). Thus, we also discuss radiation-induced or radiation-exacerbated conditions without a clear dose-dependent relationship.
Proposed Classification System for Inflammatory Skin Conditions Associated With Radiotherapy.
| Conditions induced exclusively by radiotherapy |
| Dose dependent conditions |
| Radiodermatitis (acute, chronic) |
| Conditions without clear dose dependency |
| Recall phenomenon |
| Radiotherapy-associated eosinophilic, polymorphous, pruritic eruption syndrome |
| Sclerodermiform-like panniculitis |
| Skin conditions triggered or exacerbated by radiotherapy |
| Exudative erythema multiforme, Stevens–Johnson syndrome, postradiation toxic epidermal necrolysis |
| EMPACT syndrome |
| Radiation-induced autoimmune blistering diseases |
| Radiation-induced lichen planus |
| Radiation-induced morphea |
| Other: Lupus erythematosus, neutrophilic dermatosis, GVHD, milium cysts, lichen sclerosus et atrophicus |
Abbreviation: EMPACT, erythema multiforme associated with phenytoin and cranial radiation therapy; GVHD, graft-vs-host disease
Although this review focuses on inflammatory dermatoses that follow radiotherapy, we also warn of the long-term risk for secondary tumors in the irradiated field2 and for angiosarcoma in breast cancer patients who receive radiotherapy3 as well as risk for so-called atypical vascular lesions4 and acquired lymphangiectasis.5
Because many of these conditions are probably underdiagnosed or erroneously assumed to be more widely known syndromes like radiodermatitis, it is important to be aware of their individual clinical features. Beyond the issue of diagnosis itself, we should remember that appropriate initial management of some of these conditions can improve prognosis.
Skin Conditions Induced Exclusively by RadiotherapyDose-Dependent ConditionsRadiodermatitisRadiodermatitis consists of a set of cutaneous manifestations secondary to exposure to ionizing radiation and linked to individual risk factors (Table 2), total dose received, and depth of penetration.6
Risk Factors for Radiodermatitis.
| External Factors | Patient Factors |
|---|---|
| Radiotherapy protocol | Genetic diseases |
| Total dose | Ataxia–telangiectasia, ATM heterozygosity |
| Dose fractioning | Gorlin syndrome |
| Surface and volume of application | Fanconi anemia |
| Type of radiation | Bloom syndrome |
| Xeroderma pigmentosum | |
| Radiosensitizing drugs | Connective tissue disease |
| Increased cell damage | Systemic lupus erythematosus |
| Scleroderma | |
| Rheumatoid arthritis | |
| Physical factors | Infectious diseases |
| Obesity (marked skin folds) | Human immunodeficiency virus infection |
| Smoking | |
| Malnutrition | |
| Prior actinic damage | |
| Sun exposure |
Abbreviation: ATM, ataxia telangiectasia mutated gene protein.
The acute form develops within 90 days of therapy and the chronic form appears later.
Acute radiodermatitisThe acute form generally appears in the irradiated zone an average of 10 to 14 days after radiotherapy.6 Currently, the clinical manifestations are mild to moderate in their intensity, attributable to advances in modern devices such as linear accelerators and megavoltage units that assist in delineating the radiation field (conformal radiotherapy) and in appropriately fractioning the dose.
The severity of acute radiodermatitis is usually classified in 4 levels according to the fourth version of the unified criteria of the National Cancer Institute7 (Table 3). The initial stages are marked by desquamation and limited extension. The rash then becomes progressively more exudative, extends beyond skin folds, and finally causes cutaneous necrosis and ulceration.
Acute Radiodermatitis Severity Scale According to the National Cancer Institute (Version 4).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Skin lesions | Faint erythema Dry scaling | Moderate–brisk erythema Moist desquamation | Intense erythema Pitting edema Moist desquamation | Ulceration Necrotic plaques |
| Epidermal necrosis | No | No | Superficial | Full thickness dermis |
| Location | Initially follicular | Mostly confined to skin folds | Not confined to skin folds | Not confined to skin folds |
| Other | Depigmentation, hair loss | Moderate edema | Hemorrhagic zones after trauma | Spontaneous bleeding |
| Examples |
The synergy between chemotherapy and radiotherapy in treating malignant tumors sometimes increases toxic skin effects. New targeted therapy drugs, such as anti-epithelial growth factor agents (cetuximab, panitumumab) associated with radiation usually trigger high-grade acute radiodermatitis early because these drugs have radiosensitizing effects.8 A higher rate of late cutaneous fibrosis has also been described in association with their use.9
The immediate appearance of acute radiodermatitis is the result of structural changes that occur when ionizing radiation leads to the release of free radicals that damage DNA and alter the maturation and reproduction of basal stem cells in the epidermis.10 This process alters the barrier function of the epidermis, leading to greater transdermal loss of water and partial or total epidermal necrosis. A proinflammatory environment is also generated through local recruitment of white blood cells and sustained production of interleukin (IL) 1.11 Angiogenesis, fibrogenesis, and the formation of granulation tissue also change during the process.12,13 The loss of Langerhans cells combined with a proinflammatory environment and impairment of the epidermal barrier function can invite superinfections.14
Reduced mitotic activity of the germ cells of sebaceous glands, hair follicles, and the epidermis accounts for the dryness, hair loss and other manifestations associated with radiotherapy.
Histopathology reveals epidermal changes, including vacuolar degeneration of the basement membrane and necrotic keratinocytes as well as the presence of spongiosis that can lead to subepidermal blister formation.15 Perivascular inflammatory infiltrates, dilated blood vessels with thrombi, and dermal edema can also be observed.
Recent systematic reviews of means to prevent and treat acute radiodermatitis describe management strategies in detail and include the following recommendations1,16:
- -
Wash the irradiated areas with a mild shampoo or soap to prevent superinfection.
- -
Although deodorants containing aluminum salts or pads containing metals were formerly believed to increase radiotherapeutic toxicity and were therefore contraindicated, clinical trials have demonstrated that these products do not exacerbate the severity of radiodermatitis (evidence level IA).16,17
- -
The use of topical corticosteroids, for their anti-inflammatory effect, is controversial. Medium to high potency corticosteroids are effective in treating already established radiodermatitis,18 but trials have been unable to demonstrate their efficacy in reducing the severity of radiodermatitis when used prophylactically. However, they do improve quality of life by reducing itching and burning.16
- -
If a superinfection is suspected, a bacterial culture should be started, along with topical (silver sulfadiazine cream) or systemic (antibiotic) treatment.
Special precautions should be taken with gynecologic or anal canal tumors because they are associated with grade 3 or 4 severity and greater risk of superinfection.
- -
Skin folds should be exposed to air because they are more often affected by radiodermatitis,6 Restrictive clothing, exposure to sunlight, and injury are to be avoided.
Aloe vera, trolamine salicylate creams, hyaluronic acid, and silver or sucralfate dressings have not proven useful for preventing or treating radiodermatitis.16
A lower grade of erythema was reportedly achieved in a single open-label trial of amifostine, used because of its cytoprotective role in radiotherapy and cisplatin chemotherapy.19 The trial enrolled 30 patients.
Chronic radiodermatitisThe chronic form of radiodermatitis appears months or years after irradiation. This condition must be distinguished from so-called late effects of radiotherapy, which are the result of a failure to previously cure high-grade acute radiodermatitis.
There are various stages of chronic radiodermatitis according to the classification of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer,20 whose system is based on the extension and severity of cutaneous changes (Table 4).
Chronic Radiodermatitis Severity Scalea
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Skin | Slight atrophy Some hair loss Hyper- or Hypopigmentation | Patch atrophy Total hair loss Telangiectasia | Marked atrophy Gross telangiectasia | Ulceration |
| Subcutaneous cellular tissue | Slight induration (fibrosis) and partial loss of tissue | Moderate fibrosis (asymptomatic) Field contracture <10% linear reduction | Severe induration and loss of subcutaneous tissue Field contracture >10% linear reduction | Necrosis |
| Mucous membrane | Slight atrophy Slight dryness | Moderate atrophy Telangiectasia | Marked atrophy with associated intense dryness Thick telangiectasia | Ulceration |
Source: Adapted from Toxicity Criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer, 1995.20
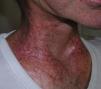
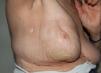
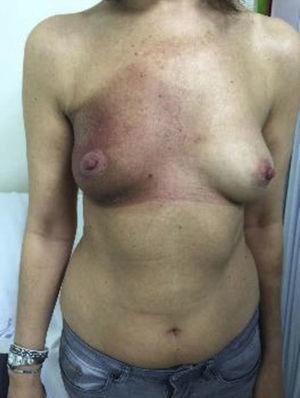
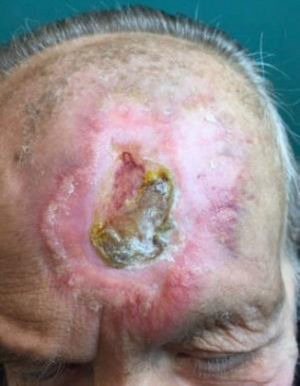
The condition presents with poikiloderma, hyper- or hypopigmented areas, atrophy, and telangiectasia (Fig. 1). Probably related to microvascular damage, telangiectasia is more common in grade 3 or higher, especially after a booster dose of radiation.21 Desquamation, hyperkeratosis, and xerosis are also common. Loss of nails, hair and skin adnexa may occur and sweating may be impaired in some parts of the body.
Localized or generalized cutaneous fibrosis is another form of presentation. Retraction and associated impedence of movement may develop. Fibrosis is triggered by various factors, but the role of transforming growth factor (TGF) ß stands out. This factor activates fibroblasts, which synthesize and secrete procollagen and other components of the extracellular matrix.22
On histology, epidermal atrophy, endothelial hyperplasia, and sclerotic or thrombosed vessels can be observed. If cutaneous fibrosis develops, the dermis and subcutaneous tissue will be replaced by fibrous tissue and atypical fibroblasts.23
Pulsed laser therapy has been reported to be useful in treating persistent telangiectasia.24 Fibrosis is the most difficult cutaneous complication to treat. Pentoxifylline has been tried at a dose of 800mg/d on its own or in combination with vitaminE.25 This peripheral vasodilator also has antifibrotic properties related to a reduction in TGF-ß expression and the phenotypic normalization of altered fibroblasts. Studies in humans that clearly demonstrate the efficacy of this approach are scarce, however.16 There are anecdotal reports of attempts to use other therapeutic modalities, such as intramuscular injections of liposomal copper/zinc superoxide dismutase 3 weeks after onset of fibrosis26 and low-dose interferon-γ based on its inhibition of dermal fibroblasts.27
Conditions Without a Clear Dose-Dependent RelationshipRecall PhenomenonA rash confined to previously irradiated skin, recall phenomenon is triggered by certain drugs, mainly chemotherapy agents. A variable amount of time (from days to years) during which the skin appears healthy, without associated radiodermatitis, may pass before signs appear.28 A minimum of 7 days between radiotherapy and drug intake is required for a diagnosis of recall phenomenon, since certain clinical pictures that emerge before that time might be triggered by radiosensitizing substances. Cases of recall phenomenon have been described up to 7 years after radiotherapy.29 The median interval, however, is 39.5 days.28
The skin is affected in 2 out of every 3 patients, but recall has also presented as pneumonitis, mucositis, and esophagitis; the central nervous system and small intestine have also been affected.28
Drugs that trigger the recall phenomenon are usually chemotherapy agents, most often taxanes (paclitaxel, docetaxel) and anthracyclines (adriamycin, doxorubicin), which together account for half the described cases. Other implicated chemotherapeutic agents are bleomycin, actinomycin D, gemcitabine, 5-fluorouracil, methotrexate, and vinblastine. Medications that are not cytotoxic, such as antituberculosis drugs,30 tamoxifen,31 antibiotics,32 and simvastatin33 have also been blamed.
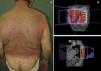
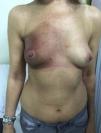
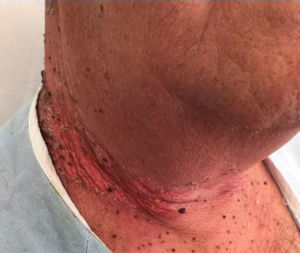
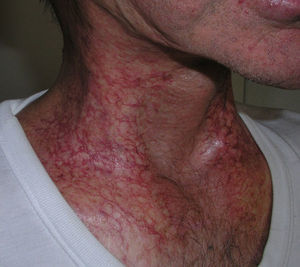
Clinical manifestations, which overlap those of acute radiodermatitis, involve an erythematous plaque that is more or less exudative and has well defined edges coinciding with the irradiated field (Fig. 2). Recall can be distinguished, however, by the fact that radiodermatitis or other lesions need not have developed previously in the irradiated field (Table 5). Depending on severity of the eruption, blisters, ulcers, hemorrhagic zones, or necrosis might develop. Signs and symptoms are more intense when the interval between the use of radiotherapy and the chemical trigger is shorter.28
Differential Diagnosis of Eruptions in Patients Exposed to Radiation.
| Radiodermatitis | Recall Phenomenon | Erythema Multiforme | SJS/TEN/SJS–TEN | |
|---|---|---|---|---|
| Extension | Irradiated field | Irradiated field | Irradiated field+dissemination | Irradiated field+dissemination |
| Rash type | Erythema/desquamation/ulceration | Erythema/desquamation/edema | Maculopapular | Maculopapular+mucositis |
| Latency period, after radiotherapy | 2 wk | 1 wk to months | 10–20 d | 10–20 d |
| Fever | Yes/no | No | Yes/no | Yes/no |
| Mechanism | Cytotoxicity | Possible hypersensitivity | Hypersensitivity | Hypersensitivity |
Abbreviations: SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
There seems to be a radiation dose threshold that must be reached for recall dermatitis to develop. Patients who received radiation on several fields at different doses have been reported to develop a recall phenomenon only on more intensely treated fields.34
Likewise, the time elapsing between therapy and the appearance of a recall phenomenon also seems to be dose dependent, as higher doses lead to more rapid reactions.28 In addition, intravenous administration of the trigger drug produces a more rapid recall phenomenon than oral administration.
Histologic findings overlap those of both acute and chronic forms of radiodermatitis. The fairly nonspecific findings described include vacuolar degeneration of the basement membrane, necrotic keratinocytes, and superficial vascular ectasia.35 A mixed inflammatory infiltrate, dermal sclerosis, and a psoriasiform pattern of dermatitis are also common.36
Pathophysiologic observations described in the context of recall phenomenon have included vascular injury in the irradiated zone31 and cellular reduction or changes in the basement membrane of the epidermis.37
Currently the most widely accepted theory holds that recall phenomenon is a hypersensitivity drug reaction35 similar to a fixed drug eruption. According to this view, it would be a nonimmunologically mediated inflammatory reaction. Supporting the theory is the high frequency of onset after first exposure to a trigger drug in HIV-infected patients with CD4 counts under 30.38 Also pertinent is the proinflammatory role of increased secretion of cytokines such as IL-1, IL-6, and tumor necrosis factor in the irradiated field. Thus, these patients develop the inflammatory response at a lower radiation threshold as soon as the trigger drug responsible for the recall phenomenon is introduced.
The cornerstone of treatment is to withdraw the trigger drug, after which the eruption should improve quickly. Systemic or topical corticosteroids and antihistamines have not been associated with rapid improvement in established symptoms.39 Reexposure to the trigger reproduces the recall phenomenon in about 75% of the cases, although the eruption is usually less intense.39 If the drug is used again, a lower dose can be given or it can be accompanied by oral corticosteroids in an attempt to prevent an inflammatory response.28
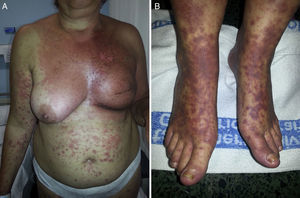
Radiotherapy-Associated Eosinophilic, Polymorphous, Pruritic Eruption SyndromeThe term eosinophilic, polymorphous, pruritic eruption associated with radiotherapy (EPPER syndrome) was first used in 1999 by Ruedaetal.40 in a study of 83 cancer patients, 17% of whom developed an extensive pruritic rash after radiotherapy. Histology of the lesions showed an abundant eosinophilic inflammatory infiltrate similar to that seen in other eosinophilic skin conditions. This clinical picture typically appears during a radiotherapy cycle or immediately afterwards, although there have been reports of cases in which the rash appeared 9 months later.41
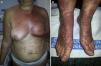
EPPER syndrome was initially described as a polymorphous condition characterized by erythematous papules and abrasions, although wheals, vesicles, firm blisters, or nodules sometimes also appear (Fig. 3). The eruption extends beyond the irradiated field and has a predilection for the extremities. Mucous membranes or palms and soles remain unaffected. Intense generalized pruritus is typical, and symptoms may last weeks or even months.40 Pustular lesions have also been described.42
The majority of patients who develop EPPER syndrome have squamous cell tumors, mainly cervical carcinoma, but this complication has also been described in patients treated for solid tumors.
The pathophysiologic mechanism is poorly understood. It has been suggested that EPPER syndrome may be a type I (immunoglobulin [Ig] E-mediated) hypersensitivity reaction involving the release of several cytokines. A typeIV hypersensitivity reaction, related to the clonal expansion of type-2 helper T cells—similar to events in systemic hypereosinophilic syndromes—has also been suggested.
A necessary finding for a pathophysiological diagnosis of EPPER syndrome is the presence of a dense, superficial and deep perivascular inflammatory infiltrate (Fig. 3) with over 10000 eosinophils/mm3. Intraepidermal vesicles with eosinophils and subepidermal blisters that are histologically indistinguishable from bullous pemphigoid may also be observed although direct immunofluorescence at the dermal-epidermal junction is negative. Necrotic keratinocytes or other signs of erythema multiforme are not found. Direct immunofluorescence only shows occasional superficial perivascular IgM and C3 deposits, but this finding is common in many inflammatory skin disorders.
A blood work-up occasionally reveals high levels of circulating eosinophils, but this finding is not characteristic. In fact, high concentrations of eosinophils in association with tumors is a well known observation43 and may be merely a casual finding in the context of EPPER syndrome.
This condition usually resolves spontaneously.
Sclerodermiform-like Panniculitis After RadiationAnother name for sclerodermiform-like panniculitis after radiation is sclerosing postirradiation panniculitis. This uncommon form of panniculitis presents as a progressive induration of subcutaneous tissue confined to the irradiated zone. Associated epidermal effects are not seen. This condition has been described in women with breast cancer treated with radiotherapy.44,45 The interval between irradiation and cutaneous sclerosis is usually 4 to 8 months, but cases have been said to develop even 7 years after radiotherapy. However, even though onset may be late, the typical signs of chronic radiodermatitis are not present. Suspicion often initially points to local recurrence of a tumor or subcutaneous metastasis.
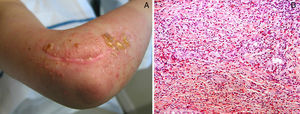
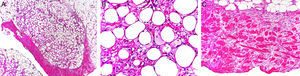
Histology shows the characteristic features of a predominantly lobular panniculitis without vasculitis.46 Lobules contain necrotic adipocytes and a dense inflammatory infiltrate of foamy histiocytes (Fig. 4). Lipophagic granulomas in the form of histiocytes surrounding necrotic fat cells may be present. Also found are thickened, sclerotic-appearing septa without an associated inflammatory infiltrate.
A, Predominantly lobular panniculitis (hematoxylin-eosin, original magnification ×100). B, Foamy histiocytes surrounding fat cells (hematoxylin-eosin, original magnification ×400). C, Sclerosis and thickening of hypodermal septa (hematoxylin-eosin, original magnification ×100).
Images provided by Dr. Luis Requena Caballero.
No specific treatment is available for this condition, which gradually improves spontaneously.
Skin Conditions Triggered or Exacerbated by RadiotherapyIsoradiotopic ResponseIt has been suggested that various skin conditions that emerge in previously irradiated fields may result from a Koebner phenomenon or a Koebner-like isoradiotopic response. However, the existence of a variable latency period between radiotherapy and the skin condition along with the absence of a Koebner phenomenon in some of these dermatoses under normal conditions has led some to assert that we are dealing with a distinct entity, namely an isoradiotopic response.47 Such a response would be a phenomenon exclusively linked to the ionizing radiation of radiotherapy, after which diverse, poorly understood mechanisms trigger a variety of dermatoses in the irradiated fields. We will now describe some of these radiation-induced dermatoses.
Erythema multiforme, Stevens–Johnson Syndrome, and Postradiation Toxic Epidermal NecrolysisIonizing radiation can induce skin disorders across the spectrum comprised of erythema multiforme, Stevens–Johnson syndrome, and toxic epidermal necrolysis. We summarize a recent review of 165 cases reported up to 2011,48 most of which met the criteria for erythema multiforme. A high percentage of the cases in this group were not associated with drugs or other known triggers. However, cases grouped in the Stevens–Johnson syndrome and toxic epidermal necrolysis categories did have clear associations with such triggers.
The culprit medications were most often antiepileptic drugs (mainly phenytoin and phenobarbital). Amifostine was the next most frequently implicated drug.
The first sign is typically a maculopapular rash that is initially confined to the irradiated zone but later spreads beyond it48,49 (Table 4, Fig. 5).
Presentations of the Stevens–Johnson or toxic epidermal necrolysis types have occasionally been reported to stay within the irradiated field.50
The median time elapsing between radiotherapy and the appearance of the rash is 28 days, but longer intervals have been reported when there is no drug trigger.48
A subgroup of patients develop the so-called EMPACT syndrome (referring to erythema multiforme associated with phenytoin and cranial radiation therapy).51 In these cases, the disease has metastasized to the brain and concomitant treatment with phenytoin and cranial radiotherapy is required. In all described cases the patients developed an erythema multiforme reaction that began in the irradiated cranial zone and later spread to the rest of the body. Radiotherapy and an antiepileptic drug are cofactors, evidenced by the fact that reintroduction of the drug by itself does not restart the skin reaction.
Pathophysiology has identified a wide-ranging group of drugs metabolized by cytochrome P450 3A that give rise to a series of reactive metabolites that are normally eliminated by enzymes like epoxide hydrolase and glutathione S-transferase. Ionizing radiation possibly causes an enzyme reaction and consequent accumulation of reactive metabolites, which will act as haptens, unleashing a hypersensitivity reaction.52 If these syndromes develop, the first step is to identify the most likely culprit drug and start symptomatic treatment or support therapy if required.
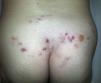
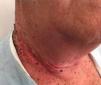
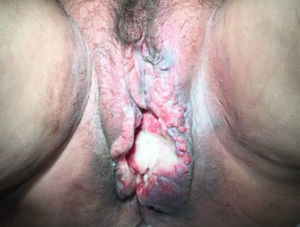
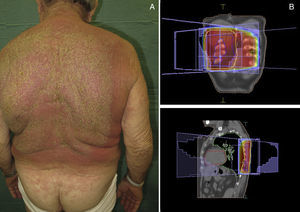
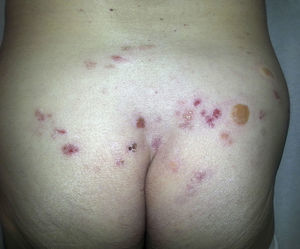
Radiation-Induced Autoimmune Blistering DiseasesOver 30 cases of bullous pemphigoid triggered by radiotherapy have been reported.53 Most blistering diseases begin with eruptions in the irradiated field (Fig. 6) and may later spread farther.54 This condition has largely been described in women who have been treated for breast cancer55; the mean age is 75 years. An interesting fact is that onset is often a year after radiotherapy ended, although some reactions have developed during or immediately after therapy.
The pathophysiologic mechanism remains unclear, although the formation of antibodies, radioinduced changes in vascular permeability, and the use of tamoxifen have been suggested causes.53
Histologic features include the characteristic subepidermal blisters with IgG and C3 deposition and elevated BP180 and BP230 titers on indirect immunofluorescence.
Pemphigus vulgaris is observed somewhat less often than radiation-induced bullous pemphigoid.56 In both conditions, flaccid blisters and erosion begin to appear in the irradiated zone and later spread beyond it. The latency period between radiotherapy and the cutaneous eruption varies from a week to a year. Histologic and immunopathologic features are the same, although additional findings in pemphigus vulgaris include areas of necrotic keratinocytes (a finding that is more common in paraneoplastic pemphigus) and reactivity to a keratinocytic antigen (110kDa) demonstrated by Western blot.56 Radiation-induced pemphigus vulgaris reactions usually respond well to conventional systemic corticosteroid or other immunosuppressant treatment.
Isolated cases of radiation-induced pemphigus foliaceus57 and paraneoplastic pemphigus58 have also been described.
Radiation-Induced Lichen PlanusLichen planus appearing exclusively in a previously irradiated field is rare.59
This complication develops within 3 months of the completion of radiotherapy generally in patients without a history of lichen planus.60 Polygonal, erythematous-violaceous papules develop in the irradiated field but may also affect other areas.61 The lesions respond well to high-potency topical corticosteroids.
The role of radiotherapy may involve changes in the cutaneous barrier with the expression of autoantigens and antigenic peptides in keratinocytes, which would be recognized by the CD8 cytotoxic lymphocytes that are fundamental in the pathogenesis of lichen planus. These changes, in addition to the release of numerous cytokines, such as IL-1, IL-6, TNF and TGF-ß1, and the release of extracellular matrix metalloproteinases that damage the basement membrane.61
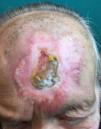
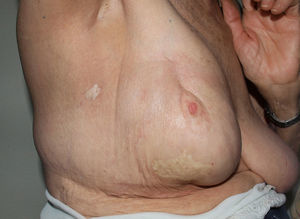
Radiation-Induced MorpheaA recent review of the literature, published in 2014, found 66 cases of radiation-induced morphea.62 Similarly to sclerodermiform-like panniculitis, this complication of radiotherapy has been reported most often in patients with breast cancer, although it has also been described in the context of other tumors.62 The typical presentation is an erythematous patch that gradually hardens (Fig. 7). Although the eruption is usually confined to the irradiated field, unlike sclerosing panniculitis it may spread beyond, particularly to the extremities.63 It does not progress to systemic disease, but sclerosis does lead to functional limitations. The interval between irradiation and the cutaneous symptoms ranges from weeks to years, although it most often appears within a year of therapy.62
Present are the typical histologic findings of idiopathic morphea: a lymphohistiocytic dermal infiltrate along with thickening and sclerosis of dermal collagen.
The pathophysiologic mechanism could be damage to fibroblasts and expression of neoantigens initially caused by radiation. These antigens would eventually be recognized by B and T cells, triggering creation of a proinflammatory environment with release of cytokines and activation of fibroblasts along with increased production of collagen.62 TGF-ß also appears to play an important role.62
The induration may improve spontaneously but, like idiopathic morphea, the radiation-induced form presents therapeutic challenges. When topical tacrolimus does not improve the symptoms, treating physicians have resorted to photodynamic therapy (psoralen-UV-A therapy or UV-B phototherapy with acitretin)64 as well as methotrexate and tetracyclines.
Other Radiation-Induced Skin ConditionsThe literature includes anecdotal reports of radiation-induced lupus erythematosus,65 Sweet syndrome,66 milium cysts,67 and lichen sclerosus et atrophicus.68
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful for the cooperation of the radiation oncology department of Hospital General Universitario Gregorio Marañón and for photographs provided by physicians mentioned in figure captions.
Please cite this article as: Hernández Aragüés I, Pulido Pérez A, Suárez Fernández R. Dermatosis inflamatorias asociadas a radioterapia. Actas Dermosifiliogr. 2017;108:209–220.