The immunophenotypic analysis of inflammatory cells in the tumor microenvironment of regressing melanomas is object of ongoing debate. On the other hand, benign halo nevi paradigmatically show marked inflammation. Characterizing these immune profiles can provide insights into the local immune response, its pathogenesis, and differential diagnosis.
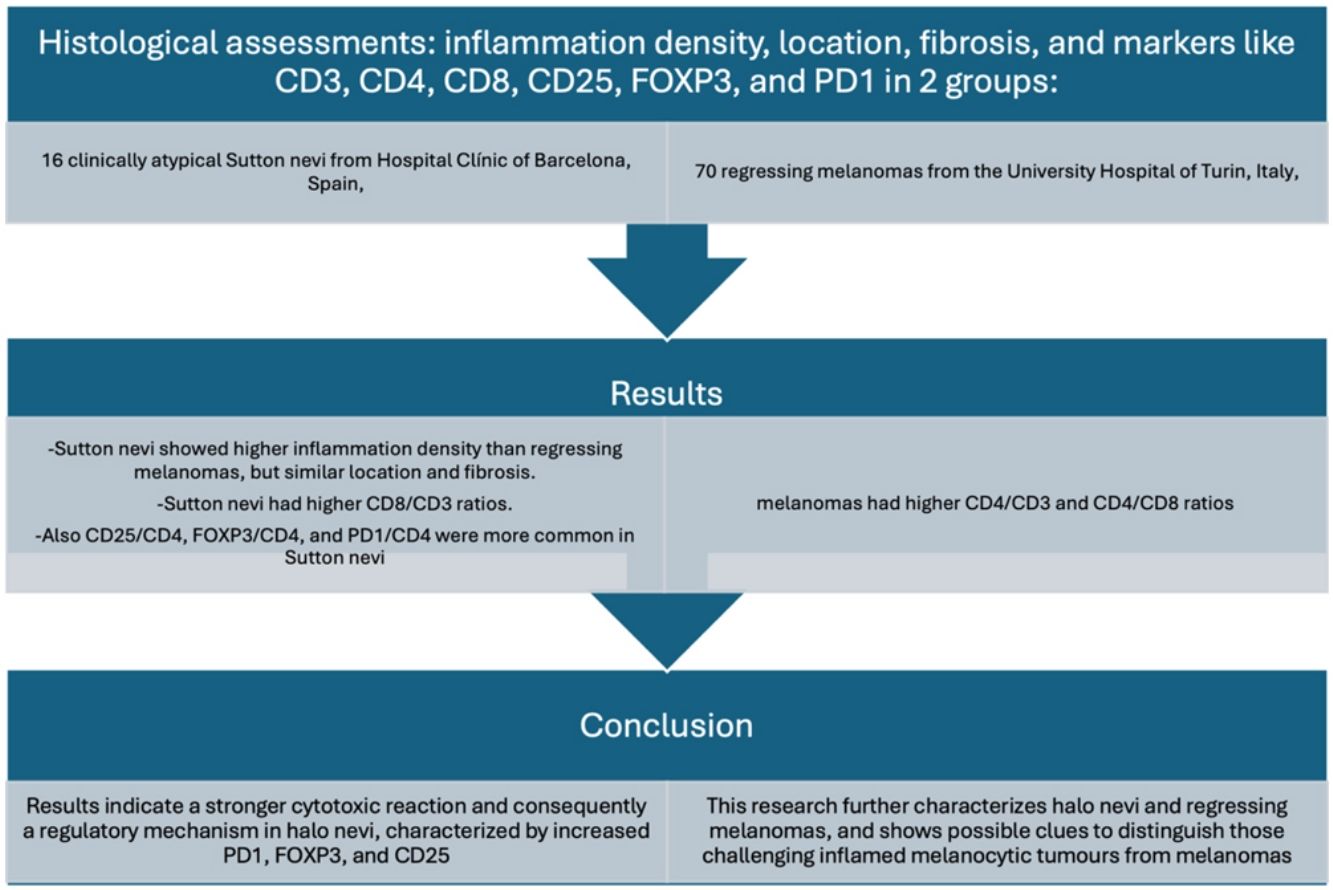
MethodologyA cohort of 16 clinically atypical Sutton nevi from Hospital Clínic de Barcelona, Spain, and 70 regressing melanomas from Turin University Hospital, Italy, were evaluated. Histological assessments analyzed inflammation density, location, fibrosis, and markers like CD3, CD4, CD8, CD25, FOXP3, and PD1.
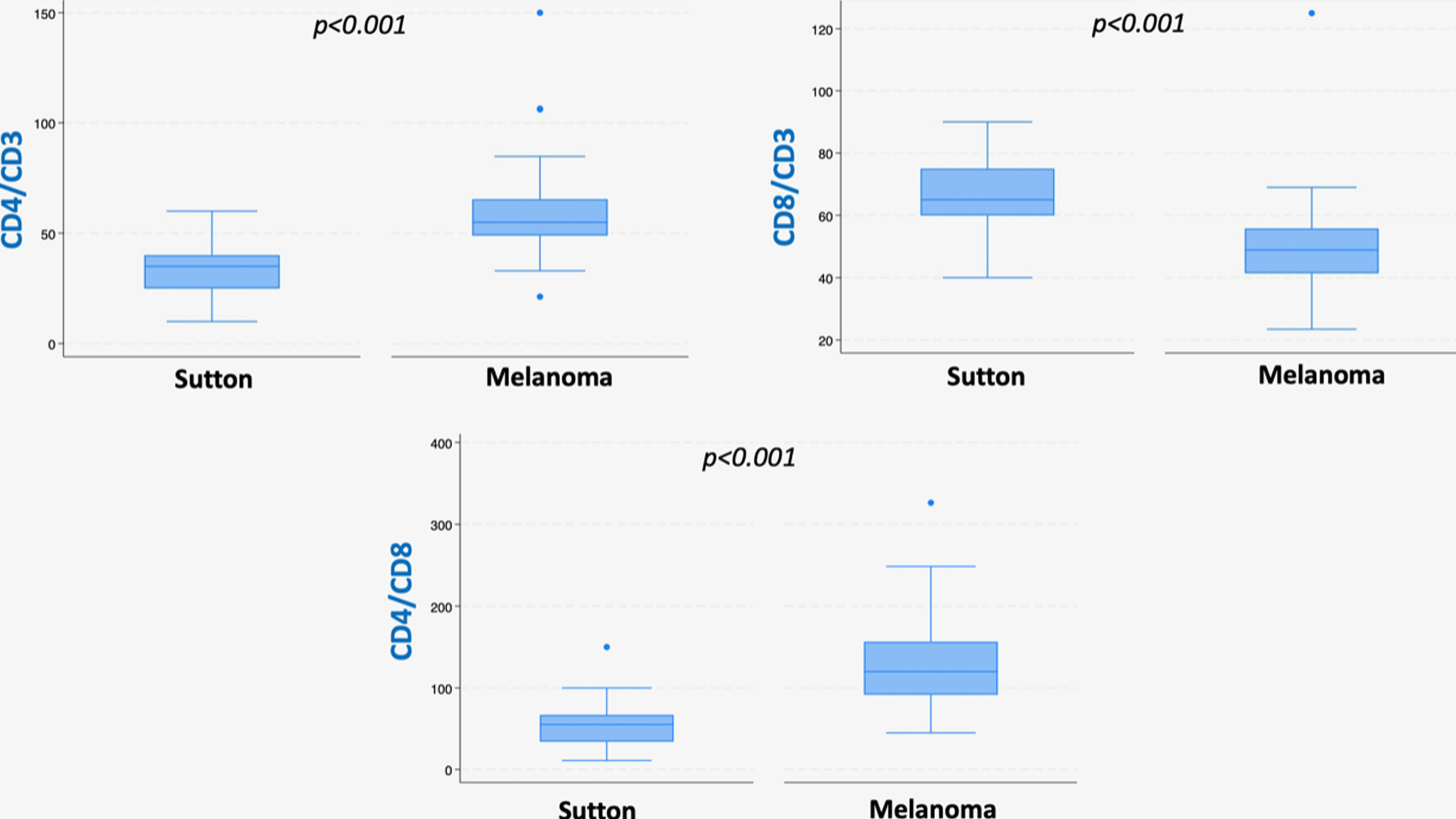
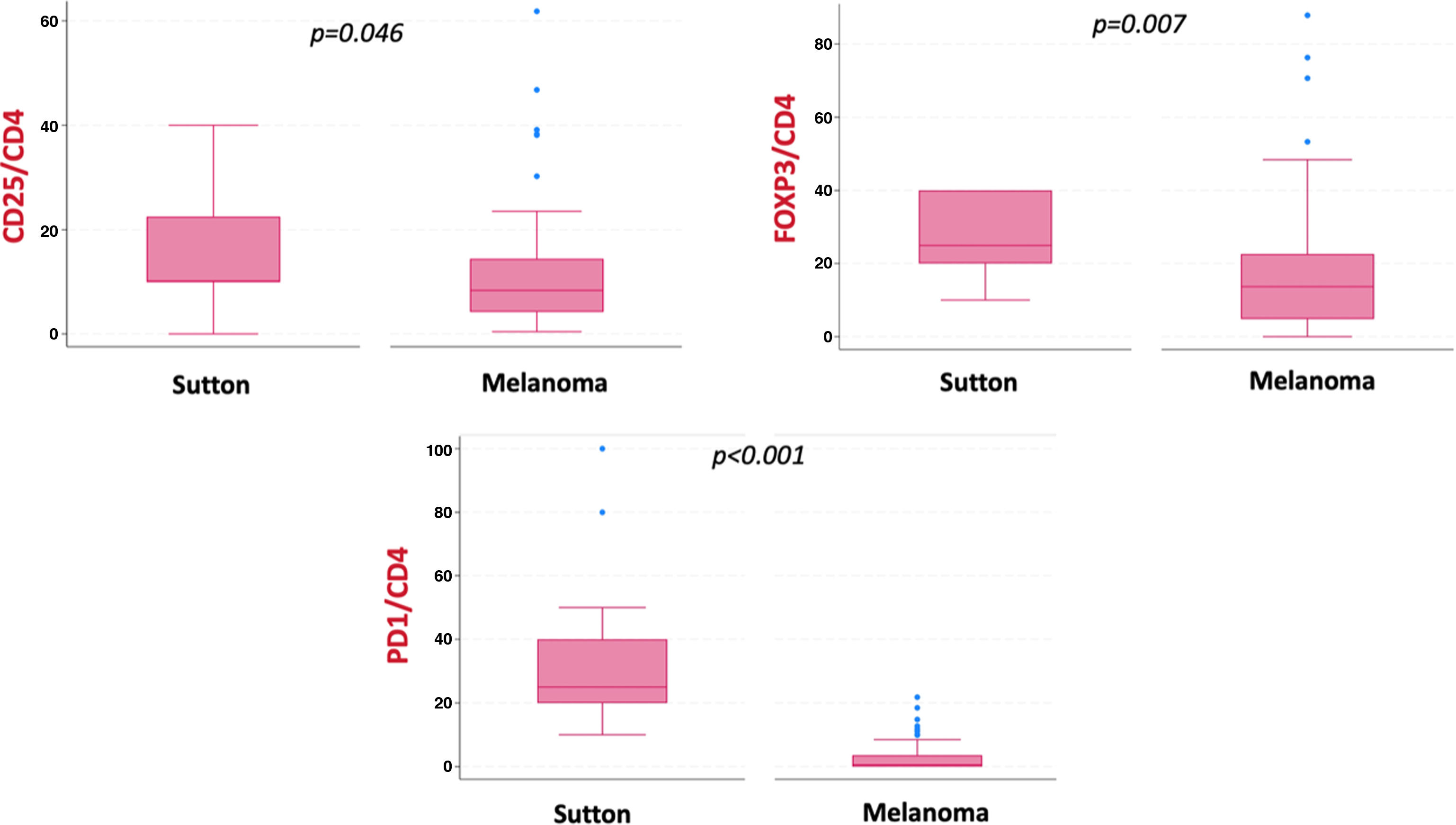
ResultsSutton nevi showed higher inflammation density than regressing melanomas, but similar location and quantity of fibrosis. Sutton nevi had higher CD8/CD3 ratios and melanomas, higher CD4/CD3 and CD4/CD8 ratios. Markers such as CD25/CD4, FOXP3/CD4, and PD1/CD4 were more common in Sutton nevi.
ConclusionResults indicate a stronger cytotoxic reaction and consequently a regulatory mechanism in halo nevi, characterized by increased PD1, FOXP3, and CD25. This research further characterizes halo nevi and regressing melanomas, and shows possible clues to distinguish those challenging inflamed melanocytic tumors from melanomas.
El análisis inmunofenotípico de las células inflamatorias en el microambiente tumoral de los melanomas con regresión histológica es objeto de actual debate. Por otro lado, los halo nevus benignos, de manera paradigmática, muestran también una inflamación marcada. La caracterización de perfiles inmunológicos en estas lesiones inflamadas puede proporcionar información sobre la respuesta inmunitaria local, la patogénesis y su diagnóstico diferencial.
MetodologíaSe evaluó una cohorte de 16 nevus de Sutton clínicamente atípicos del Hospital Clínic de Barcelona, España, y 70 melanomas con regresión del Hospital Universitario de Turín, Italia. Los evaluadores analizaron la densidad de la inflamación, su localización, la fibrosis y los marcadores CD3, CD4, CD8, CD25, FOXP3 y PD1.
ResultadosLos nevus de Sutton mostraron una mayor densidad inflamatoria que los melanomas con regresión, pero similar localización y cantidad de fibrosis. Los nevus de Sutton presentaron mayores ratios de CD8/CD3, mientras que los melanomas presentaron índices más altos de CD4/CD3 y CD4/CD8. Marcadores, como CD25/CD4, FOXP3/CD4 y PD1/CD4, fueron más comunes en los nevus de Sutton.
ConclusionesLos resultados indican una reacción citotóxica más fuerte y, consecuentemente, un mecanismo regulador en los halo nevus, caracterizado por un aumento de PD1, FOXP3 y CD25. Este artículo caracteriza más a fondo los halo nevus y los melanomas con regresión y plantea posibles pistas para distinguirlos entre ellos.
Malignant melanoma is a type of skin cancer that represents a growing concern in public health due to its increasing incidence rate and metastatic potential.1,2 Moreover, the improvement in dermatological imaging modalities to identify early and difficult to diagnose melanomas has led to more pathological challenging tumors being detected.3 The study of specific features, such as tumor regression and inflammatory infiltrates, has sparked considerable interest since they could lead to misdiagnosing melanocytic lesions under the microscope.4 Therefore, it is very important to understand the immunological mechanisms involved in these phenomena, as they can aid in better diagnosis and be very useful for the development of specific targeted therapies.5,6 Tumor regression, a phenomenon observed in a significant fraction of melanomas (frequency ranges between 10% and 58%) is characterized by partial or complete reduction of the melanocytic component accompanied by a variable inflammatory infiltrate, melanophages, new vessels, and a certain degree of fibrosis.7 The influence of regression on the prognosis of melanoma has sparked extensive debate over the years.8 Some studies consider regression a negative prognostic factor, as it might have interfered with the accurate measurement of melanoma thickness, thereby affecting the proper tumor staging. Other studies suggest that histological regression increases the risk of metastasis. Conversely, some reports propose that regression does not alter prognosis, and in certain cases, it may even act as a protective factor.9–11 Studies have revealed that the distinct malignancy and biological characteristics of cancer are impacted not only by its genetic abnormalities but also by the interaction between cancer cells and their surrounding microenvironment. The interplay between the tumor and the host's immune system is particularly captivating, especially given the substantial clinical effectiveness displayed by drugs directed at these specific pathways.12 Indeed, the presence of dense tumor infiltrating lymphocytes has been associated with a better response to immune checkpoint inhibitors.13 Similarly, Sutton Nevi (SN), which are benign lesions clinically characterized by an achromic halo surrounding the melanocytic nevus, have also shown associations with the activation of local immune responses.6,14 The halo phenomenon, manifested as a depigmented halo primarily associated with acquired melanocytic nevi, can also be seen in other melanocytic skin tumors, with melanoma posing heightened concern.14 In this scenario, reflectance confocal microscopy has emerged as a potential tool to differentiate benign inflamed nevi from melanoma.15 Integration of the patient's age and meticulous examination of the dermo-epidermal junction using reflectance confocal microscopy has been suggested to improve diagnostic accuracy.15
Recently, the immunophenotypic analysis of inflammatory cells present in the tumor microenvironment of regressing melanomas and halo nevi has gained increasing interest.9,16 Identifying and characterizing the immunophenotypic profile in these lesions could provide new insights into the local immune response and its potential implications in the pathogenesis and progression of these cutaneous entities. The characterization of immune infiltrate in melanomas with histological regression revealed a prevalence of effector and suppressor T cells, B cells, natural killer cells, macrophages, and regulatory dendritic cells, all capable of exerting contrasting immuno-stimulatory or immunosuppressive effects within the tumor microenvironment.10 Conversely, the inflammatory infiltrate observed in halo nevi stands out due to a higher quantity of active cytotoxic T cells and PD1-positive regulatory T cells vs the infiltrate observed in regressing melanoma.9 In this research, we aim to examine the immunophenotypic profile of inflammatory cells present in halo nevi through a detailed analysis of histological markers, comparing them to a recently published cohort of melanomas with regression, to enhance a deeper understanding of the immunological mechanisms linked to these skin lesions.
Materials and methodsWe conducted a retrospective cohort of 70 melanomas demonstrating histological regression diagnosed at Turin University Hospital from January 2003 through December 2014 and compared it with a prospective cohort including 16 dermatoscopically atypical Sutton nevi, excised at Hospital Clínic de Barcelona from January 2017 to December 2020. In this last group, we included those dermoscopic atypical melanocytic lesions that were excised to rule out melanoma and later histologically diagnosed as Sutton nevi. Histological assessment included degree and location of the inflammatory infiltrate within the lesion, degree of fibrosis, and the evaluation of specific immunohistochemical markers—CD3, CD4, CD8, CD25, FOXP3, and PD1—in both cohorts. All patients gave their informed consent allowing the use of their biological samples in a biobank. The study received approval from both local ethics committees at HospitalClínic de Barcelona and Turin University Hospital. The nevi group included only those nevi that displayed atypical dermoscopic features accompanied by histological regression or intense inflammatory phenomena. In contrast, nevi exhibiting exclusively histological features of regression were not analyzed. To ensure a comprehensive evaluation of histological parameters, only samples that completely removed the tumor and included the entire thickness of the melanocytic lesion in the examination were considered. Consequently, shave and punch biopsies, partial excisions, and samples with inadequate material were excluded from the study. The patients’ demographic, clinical, and pathological features were documented and updated in an internal database.
For all cases, histopathological features were assessed on sections stained with hematoxylin and eosin (H&E) by 2 dermatopathologists (LC and RS). This evaluation included degree of inflammatory infiltrate, classified as low (<30%), medium (30–60%), or high (>60%), pattern/localization of the inflammatory infiltrate (diffusely infiltrative, patchy perivascular or intratumoral, underneath the tumor), and degree of fibrosis (categorized as absent, low<30%, moderate 30–60% or high>60%). In instances of disagreement, a third dermatopathologist (APC) was consulted for resolution. In the melanoma group, to distinguish from tumor-infiltrating lymphocytes (TIL), the primary criterion for defining regression was the necrosis, apoptosis or complete disappearance of malignant tumor cells, with evident partial or total active breakdown of melanocytic nests, replaced in the early phase by inflammatory cells, and in the late phase by fibrosis with dilated vessels, and melanophages.17 Regions of ulceration or those closely related or adjacent to it were excluded from consideration.
Surgical specimens were fixed in 4% buffered formalin, routinely processed and paraffin embedded. For each case, 3μm thick paraffin sections collected on Superfrost Plus slides were tested by immunohistochemistry. Immunohistochemical analysis was performed using an automated slide-processing platform (Ventana BenchMark XT Autostainer; Ventana Medical Systems, United States). The entire halo nevus cohort was stained with CD3 (Confirm anti-CD3, clone 2GV6; Ventana, Roche); CD4 (Confirm anti-CD4, clone SP35; Ventana, Roche); CD8 (Confirm anti-CD8, clone SP57; Ventana, Roche); CD25 (clone 4C9; Cell Marque, Sigma Aldrich); FOXP3 (clone 236A/E7; eBiosciences); and PD1 (clone NAT 105; Cell Marque, Sigma–Aldrich).
Lymphocyte subset populations were investigated by assessing the immunohistochemical expression of CD3, CD4, CD8, FOXP3 and PD1. For each marker, the number of positive cells per high power field (HPF) was counted manually. Each HPF corresponds to a high magnification microscope field (400×) measuring 500μm in diameter on a 40×/0.65 lens mounted on an Olympus microscope model BX41 (Olympus, Japan). Counts were made in at least 4 (max 8) HPF fields for each type of area and the total absolute value was normalized to 1mm2 (4 HPFs correspond to 1mm2). Additionally, the following ratios were calculated for every lesion: CD4+/CD3+, CD8+/CD3+, CD4+/CD8+, CD25+/CD4+, FOXP3+/CD4+, PD1+/CD4+.
Descriptive statistics summarized patient and lesion characteristics. The Mann–Whitney test compared non-normally distributed continuous data between 2 groups. Fisher's exact test examined associations in paired nominal data, particularly for small sample sizes. The chi-squared test assessed associations in larger paired nominal datasets. p-Values<0.05 were considered statistically significant. Statistical analysis was performed using Stata/SE.v.18 (StataCorp, College Station, TX, United States).
ResultsA total of 39 of the 70 patients with histologically regressed melanoma (56%) were men and 31 (44%), women, with a median age of 58 years (range, 23–78).10 Primary tumors were located on the trunk in 58.6% of cases (41/70), followed by the lower limbs (22/70, 31.4%), upper limbs (4/70, 5.7%), and head/neck (3/70, 4.3%). The most common histological type was superficial spreading melanoma, observed in 80% of cases (56/70). The mean Breslow thickness was 2.73mm (SD, 2.10). Ulceration was present in 33% of lesions (23/70). A mitotic count>1/mm2 was observed in 61% (43/70), with 38.5% (27/70) displaying diffuse neo-angiogenesis and 48.6% (34/70) exhibiting vascular invasion. TILs were described as ‘brisk’ in 28.6% of cases (20/70). Metastatic involvement of the sentinel lymph node (SLN) was noted in 37% of patients (26/70).
As for the Sutton nevi population, a total of 16 cases were suitable for analysis. The patients’ median age was 30 years (range 16–53). Among the 16 individuals, 11 were men (68.75%) and 5 were women (31.25%). Lesions were most commonly located in the interscapular region (4/16, 25%), followed by the lumbar and scapular areas (3/16, 18.8% each). The shoulder and abdomen areas each accounted for 2/16 (12.5%) of the cases. Other locations, such as the retro-auricular and paravertebral areas, each accounted for 1/16 (6.25%) of the total number of lesions. The sizes of the atypical Sutton nevi varied, with a median size of 5.5mm (range, 2–10mm). The most frequently recorded sizes were 5mm (25% of the cases), 4mm (12.5%), and 7mm (12.5%). Clinically palpable lesions were present in 4 out of 16 cases (25%). Dermoscopically, 8 out of 16 cases (50%) showed a reticular-globular pattern, while 4 out of 16 (25%) displayed a reticular pattern, and 4 out of 16 (25%) a globular pattern. According to the commonly used 7-point checklist for melanocytic lesions, a score≥3 was recorded in 15 cases (93.75%), while a score<3 was recorded in 1 case only (6.25%).18 As for the confocal features according to the Barcelona score (e.g., threshold for ruling out melanoma=0), the most common score was 1 (8/16, 50%), followed by score 0 (6/16, 37.5%) and score 2 (2/16, 12.5%).15
At histopathologic evaluation, 8 out of 16 cases (50%) exhibited a high inflammatory infiltrate (>60%). In 7 out of 16 cases (43.75%), the infiltrate was moderate (30–60%), and in the remaining case (1/16, 6.25%), it was low (<30%). No cases showed diffuse fibrosis; however, 14 out of 16 cases (87.5%) exhibited low (9/16, 56.25%) or moderate (5/16, 31.25%) fibrosis, with fibrosis being absent in 2 out of 16 cases (12.5%). The inflammatory localization pattern was diffusely infiltrative in 7 out of 16 cases (43.75%), patchy perivascular or intratumoral in 6 out of 16 cases (37.5%), and located underneath the tumor in 3 out of 16 cases (18.75%).
As for the comparative analysis between the 2 groups in terms of immuno-staining, the atypical Sutton nevi group exhibits significantly higher inflammation density than the group of melanomas with regression (p=0.008 for low-density and p<0.001 for high-density inflammation, respectively). However, no differences were observed in terms of inflammation location within the lesion (diffuse, underneath the melanocytic lesion, or patchy). Regarding the quantity of fibrosis, no significant differences were observed between the 2 groups (p>0.05). However, when assessing the quality of fibrosis, the Sutton nevi group predominantly exhibited a mild-delicate fibrosis, whereas the melanoma group displayed well-established fibrosis, characterized as sclerotic, desmoplastic, lamellar, or compact.
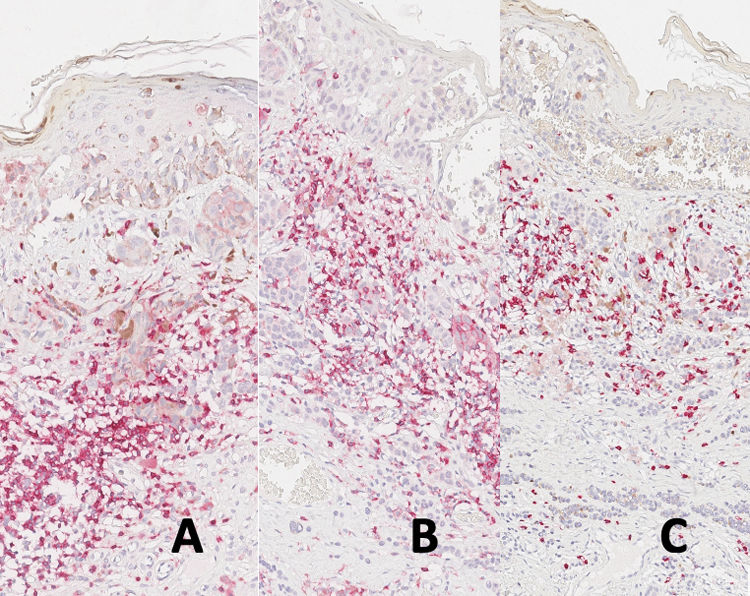
As for immunostaining evaluation, atypical Sutton nevi demonstrate a higher CD8/CD3 population ratio, while melanomas with regression showed higher CD4/CD3 and CD4/CD8 ratios (all statistically significant at p<0.05) (Table 1, Figs. 1 and 2A–C).
Among other immunohistochemical markers, CD25/CD4, FOXP3/CD4, and PD1/CD4 ratios were statistically higher in atypical Sutton nevi (p<0.05) (Table 1, Fig. 3).
DiscussionHistologically, a halo nevus exhibits a dense lymphocytic infiltrate that gradually obscures and replaces nevus cells. Similarly, regression in melanoma shares this feature, showing a dense lymphocytic infiltrate and areas where neoplastic cells have disappeared entirely. Conceptually, regression and halo nevi both demonstrate an active immune response against the melanocytic lesion.
The immunophenotyping of inflammatory cells accompanying regression in melanoma and Sutton nevi has been poorly studied. In 2015, a study by Botella et al. found a higher cytotoxic reaction in halo nevi vs melanomas with regression, validating our study, which showed a higher CD8/CD3 ratio in the Sutton nevus group.9 Similar results were found in a series of spitzoid melanocytic lesions, when these lesions were grouped into benign/low-grade tumors and malignant/high-grade tumors.16 However, the study by Botella et al. also observed a denser infiltrate of PD1-marked cells in Sutton nevi yet found no significant differences in FOXP3 expression. Conversely, our study did find significant differences, with higher PD1 and FOXP3 presence in the Sutton vs the melanoma group.9
These findings suggest an active regulatory mechanism alongside the cytotoxic response in Sutton nevi. The combined evidence of a denser infiltrate of PD1, FOXP3, and CD25 points toward a more active immune environment, aiding in better immune response regulation, especially in Sutton nevi.19
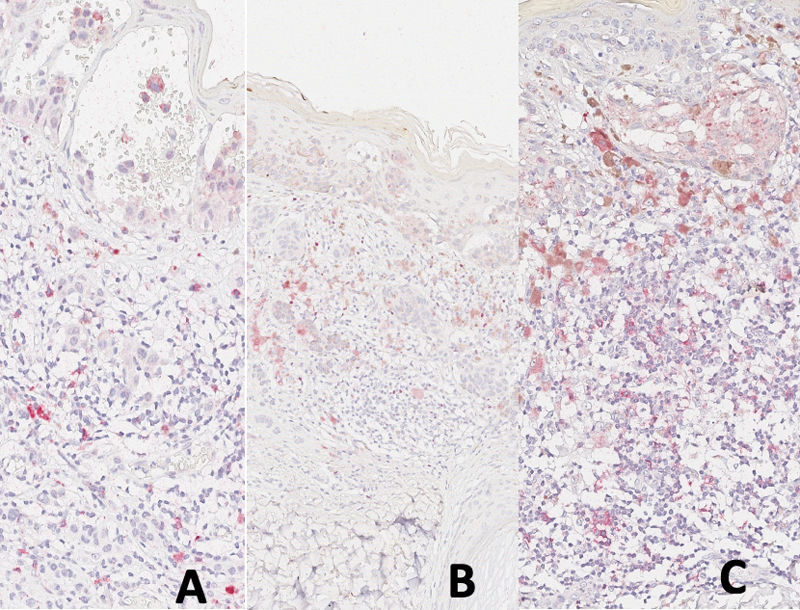
Ribero et al. suggested that in melanomas, certain neoplastic clones might evade the immune response, leading to unrestrained growth.11 In this scenario, the inefficiency of the cytotoxic response in melanomas could be linked to a decrease in CD8, as well as a reduction in the regulatory immune environment marked by PD1, CD25, and FOXP3 (Fig. 4A–C).
Recent encouraging findings from Cho et al. suggested that a compound named Celastrol exhibits significant efficacy in inhibiting tumor growth in melanoma.20 This effect is achieved by blocking the binding between IL-2 and CD25, thereby enhancing the antitumor activity of T cells. This could be very promising especially in combination with other targeted therapies for advanced melanomas.21,22
In melanomas, the presence of FOXP3+ cells has been associated with a reduction of both the innate and the acquired immune response against the tumor, employing a mechanism typically involved in shielding the body against autoimmunity.20,23,24 When we assessed the FOXP3/CD4 ratio in our study, we noticed higher levels of expression in Sutton nevi and lower levels in regressed melanomas. This discrepancy suggests a distinct state of immune tolerance between Sutton nevi and regressed melanomas, potentially serving as an early indicator of immune system deactivation in melanomas. This observation aligns with evidence indicating that in metastatic melanoma, FOXP3+ immune cells infiltrating the tumor could serve as a potential predictive biomarker for treatment response and overall survival. Balatoni et al. demonstrated that the density of FOXP3+ cells infiltrating lymph node metastases was the strongest predictor of response to ipilimumab treatment.25
This study focuses on 2 challenging entities for differentiation in our daily practice: halo nevi and malignant melanoma with regression. The autoimmune phenomena seen in Sutton nevi could induce reactive atypia, complicating its differentiation, especially for expert pathologists.26 Our aim was to describe the immune reaction in both entities: firstly, the inflammatory infiltrate in halo nevi is more active, whereas there's a pronounced cytotoxic response in Sutton nevi. Secondly, a regulatory mechanism parallel to the cytotoxic response with a denser infiltrate of PD1, FOXP3, and CD25 is observed. In conclusion, studying the immune profile of melanocytic tumors might be useful in cases of overlapping morphological features, which make it challenging to distinguish between melanoma with regression and Sutton nevus. However, the present results do not allow extrapolating the utility of the immunophenotype of inflammation to differentiate melanoma from melanocytic lesions with regression. The limitations of our study include the subjective nature of the immunohistochemistry evaluation method. Additionally, the small sample size in both groups limits the study robustness, with the sample size of the Sutton nevus group being significantly smaller vs that of the melanoma group as the diagnosis of Sutton nevi is usually clinical/dermoscopic and does not require surgical excision. The cases included in the study were those Sutton nevi that exhibit atypical clinical features, requiring excision and posing a diagnostic challenge. Such specific cases are less common in our routine clinical practice. Another limitation is that only atypical clinical presentations of Sutton nevi were included, which may limit the generalizability of the results to all types of Sutton nevi. None of these cases have recurred to date.
Understanding the composition of the immune infiltrate in cases where melanomas fully or partially disappear is crucial in an era of treatments designed to enhance the immune response vs this tumor. Based on our findings, we recommend further studies to deepen the understanding of the immunophenotype of Sutton nevi and the mechanisms underlying histological regression in melanomas, thus contributing to advancements in clinical and pathological knowledge.
Institutional review board statementThe study was conducted in full compliance with the Declaration of Helsinki, and reviewed and approved by the Research Ethics Committee of Hospital Clínic de Barcelona IRB; approval HCB/2018/0872 (June 15th, 2022).
Informed consent statementInformed consent was obtained from all subjects involved in the study.
Conflict of interestThe authors declare that they have no conflict of interest.
Data availability statementData are available upon request to the authors.