Hemangioma is the most common benign tumor in childhood, with a prevalence of 10%to12% in the first year of life.1 This vascular tumor appears in the early months of life and has 2phases: a proliferative phase of rapid growth in the first few months, followed by a slow, involuting phase that can last years.2 Tumors in certain sites require early treatment to prevent sequelae; for instance, periocular tumors have been associated with complications such as amblyopia, asymmetric astigmatism, proptosis, strabismus, and exposure keratitis.3–5
The classic treatment of hemangiomas has essentially been systemic corticosteroids.6 Other treatments used are topical and intralesional corticosteroids, laser therapy, surgery, and interferonα1. In2008 Léaute-Labréze7 described the treatment of hemangiomas with propanol, which is currently one of the most effective alternatives. In recent years timolol has been successfully used for certain hemangiomas.3,8–10
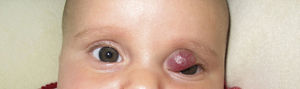
We describe the case of a 2-month-old preterm infant who, at 2weeks of life, developed a focal hemangioma of infancy on the upper left eyelid; the hemangioma blocked the child's vision and exerted moderate pressure on the eyeball (Fig. 1). The patient was assessed by the ophthalmology department and other eye conditions were ruled out. Because treatment was needed to prevent complications but the parents declined systemic treatment, timolol0.1% ophthalmic gel was applied twice daily. The gel was instilled into the palpebral conjunctiva by everting the eyelid slightly and applied to the outer surface of the hemangioma. The nasolacrimal duct was then occluded at the punctum to achieve stronger local activity and lower systemic absorption.
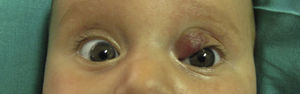
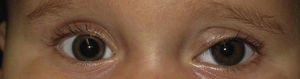
The tumor shrunk noticeably in the first week of treatment, leaving the pupillary area free; the proliferative component was also greatly reduced (Fig. 2). After 4months of treatment, the hemangioma had disappeared almost entirely (Fig. 3), with no local or systemic adverse events during treatment. By5months post-treatment, the hemangioma had not recurred.
Oral propranolol is currently one of the most commonly used drugs used to treat hemangiomas.2 Its therapeutic effects are achieved through several mechanisms: vasoconstriction and decreased expression of vascular endothelial growth factor (VEFG) and fibroblast growth factor (bFGF).8 Although propranolol is considered safe, its use has been associated with adverse reactions, such as bronchospasm, arrhythmias, bradycardia, hypotension, and hypoglycemia.3,4,9 Traditionally, systemic corticoidsteroids have been the first-line treatment for hemangiomas; however, their prolonged use has been associated with adverse effects, including hypertension, glaucoma, myopathy, and decreased weight and height gain.6 Other therapeutic alternatives, for instance, intralesional corticosteroids, can cause complications, including deformation of the eyelid, elevated intraocular pressure, and occlusion of the central retinal artery.3
Timolol is a noncardioselective beta blocker similar to propranolol. The drug is available is several forms: 0.5%eye drops,3 0.5%gel8 (not sold in Spain), and 0.1%gel (used in this case). In this form, the pharmacokinetic data suggest almost no systemic exposure, with plasma concentrations below the quantitation limit (QL=0.8ng/mL). However, the contraindications and adverse effects of systemic beta blockers should be taken into account, although side effects are rarely observed after ocular instillation.
Nine cases of palpebral hemangioma treated with timolol have been described to date. The condition was first reported in 2010 by Guo and Ni3 and more recently by Khunger and Pahwa,9 who described a hemifacial hemangioma associated with PHACE (posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities) syndrome, and by Nietal,10 who published a series of 7cases. All of these authors reported satisfactory outcomes. However, in the 2010 study by GuoandNi3 and the 7cases reported subsequently by Nietal,10 response to treatment was slower than in our patient, occurring 4to5 weeks after start of treatment. This difference may be attributable to the fact that we used a gel formulation and also applied the drug to the conjunctival surface.
In our case, timolol gel0.1% was shown to be a safe and effective treatment and we believe that its use at certain sites, such as the periocular region, could be an effective option that should be considered when systemic treatment is contraindicated.
Fernández- Ballesteros MD, et al. Hemangioma infantil palpebral tratado con timolol gel. Actas Dermosifiliogr.2012;103:444-6.