The approach to the ‘oldest-old’ patients (patients older than 85 years-of-age) with cutaneous squamous cell carcinoma (cSCC) must be adapted to the unavoidable presence of comorbidities, the inevitable decline of the physical and cognitive ability for self-support and the limited life-expectancy.1
cSCC is a potentially life threating tumor that preferentially affects the ‘oldest old’,2 a fact that often instigates individualized approaches. For primary cSCC first-line treatment is surgery either with clinically predefined margins or Mohs.3 However, the increasing frailty of these patients coupled with life-expectancy considerations substantially limit the applicability of radical, curative surgery, increasing the attractiveness of less-invasive, yet effective, therapeutic modalities.4
Herein, we compile the preliminary findings of an open-label study on the treatment of locally confined cSCC in four patients over 85 years old with individualized, patient- and tumor-adapted immunocryosurgery modifications.
Patients were scheduled to be treated with an extended and intensified immunocryosurgery treatment protocol that included a more intense cryosurgery session (open spray liquid N2, 2 freeze-thaw cycles, 30s each vs 15s for BCC5) on days 14 and 28 of daily imiquimod application. Once daily imiquimod was to be resumed already on the same night and to be continued either for three more weeks (‘5/1 scheme’) or until the next cryosurgery session on day 28 and then for three more weeks (7 weeks imiquimod/2 cryosurgery sessions: ‘7/2 scheme’). Addition of optional adjuvants (intralesional methotrexate, tretinoin cream) was decided during the bi-weekly treatment course evaluation. Tretinoin cream 0.05% was added at week 2 only if minimal topical inflammatory tumor response was evident. Likewise, intralesional methotrexate was scheduled for the days of the cryosurgery sessions, yet with anticipated individualized adaptation. Treatment end and scheduled visits were planned as previously.5
Eight biopsy-confirmed cSCC in four patients (3 males: 1 female, median age: 88 years [range: 85–89]) were treated on immunocryosurgery basis (Table 1). Different tumor-treatment trajectories with individualized adaptations of the prototype therapeutic scheme are presented below.
Clinical characteristics and therapeutical parameters of 8 cutaneous squamous cell carcinomas treated with immunocryosurgery (±adjuvants).
| Tumor | Patient | Age/gender | Localization | Diameter (mm) | Prior surgery | Treatment cycles | Imiquimod (weeks) | Cryo sessions | Adjuvants | Outcome | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 87 Male | Scalp | 20 | 10 years ago | 2 | 1st cycle: 7; 2nd cycle: 5 | 1st cycle: 2; 2nd cycle: 1 | No | Clearance | 42 |
| 2 | 2 | 85 Female | Cheek, left | 15 | No | 1 | 7 | 2 | Trea; 2xMTXb (5mg) | Clearance | 14 |
| 3 | 2 | Temple, left | 11 | No | 1 | 7 | 2 | Tre; 2xMTX (5mg) | Clearance | 14 | |
| 4 | 2 | Cheek, right | 8 | No | 1 | 5 | 1 | Tre | Clearance | 12 | |
| 5 | 2 | Nose, left | 7 | No | 1 | 5 | 1 | Tre | Clearance | 12 | |
| 6 | 3 | 87 Male | Scalp | 30 | No | 2 | 1st cycle: 5; 2nd cycle: 5 | 1st cycle 1; 2nd cycle: 1 | 1st cycle: 1xMTX (5mg); 2nd cycle: None | Clearance | 12 |
| 7 | 3 | Scalp | 25 | No | 1 | 7 | 2 | 1xMTX (5mg) | Clearance | 3 | |
| 8 | 4 | 89 Male | Cheek, left | 22 | No | 2 | 1st cycle: 9; 2nd cycle: 5 | 1st cycle: 3; 2nd cycle: 2 | 1st cycle: 3xMTX(5mg); 2nd cycle: 2xMTX(5mg) | Partial response-relapse | 8 |
Abbreviations.
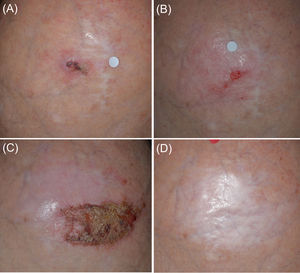
Patient 1 (Fig. 1) was initiated with a ‘7/2 immunocryosurgery cycle’ with a partial tumor response (Fig. 1B). The tumor was eradicated with an additional, modified, ‘5/1 immunocryosurgery cycle’ (5-weeks imiquimod with an additional cryosurgery session at the last, 35th cycle's day, Fig. 1C) and remained relapse free 30 months thereafter (Fig. 1D).
Panel (A): Initial presentation of the Tumor 1 in the mid-scalp (Patient 1). The tumor had a maximal diameter of 20mm and was a relapse from a previous surgery 10 years ago. Panel (B): After the initial ‘7/2 treatment cycle’ (7-weeks of imiquimod and cryosurgery at days 24 and 28 of the treatment cycle) a partial response of the tumor was achieved. Panel (C): The tumor area after the second treatment cycle of 5 weeks imiquimod and 1 cryosurgery session (‘5/1 cycle’). Panel (D): Sustained complete clearance at 30-month follow up.
Patient 2 initially presented with two cSCC (Tumors 2 and 3) which responded completely after parallel treatments with ‘7/2 immunocryosurgery cycles’ and two intralesional methotrexate injections (2.5mg pro tumor and pro session at the cryosurgery days). Two smaller cSCC (Tumor 4 and 5) diagnosed during follow-up were treated with one standard ‘5/1 immunocryosurgery cycle’ each and adjuvant tretinoin cream. Relapses were not recorded for at least 12 months.
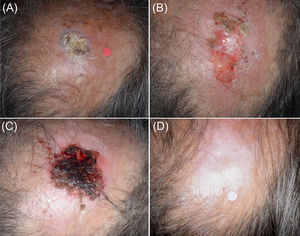
Patient 3 (Fig. 2) developed an intense inflammatory reaction after the first cryosurgery session of the ‘7/2 immunocryosurgery cycle’ with adjuvant 5mg intralesional methotrexate and did not consent to it at the 2nd cryosurgery session at week 4. Instead, an additional week of imiquimod was scheduled to complete a ‘5/1week’ immunocryosurgery cycle’. The tumor responded partially (Fig. 2B) and at one-month follow-up a second ‘5/1 immunocryosurgery cycle’ without adjuvants resulted to complete tumor clearance (Fig. 2C) that persisted after 12 months follow up (Fig. 2D). At six months follow-up a second cSCC was diagnosed and treated with a ‘7/2 immunocryosurgery cycle’ with adjuvant methotrexate at the first cryosurgery session (Day 14). Subsequently the patient was lost to follow-up.
Panel (A). Initial presentation of the Tumor 6 (Patient 3) in the midscalp. An intense inflammatory response after the first cryosurgery session of the ‘7/2 treatment cycle’ Panel (B). Tumor rests at the end of the first treatment cycle. Only partial response was noted. Panel (C). The tumor area at the end of the second treatment cycle. Panel (D) The tumor site at the 12-month follow-up. The healing process is excellent in an area that normally requires complex surgical reconstruction procedures.
Patient 4 presented with a rapidly growing cSCC on his left cheek (Table 1). He initially denied surgical excision or radiotherapy. However, the neoplasm responded only partially to 14 weeks imiquimod application, 5 sessions of cryosurgery and 5 intralesional methotrexate injections (Table 1). Subsequently he consented to radiotherapy and remained tumor free at the six months follow-up.
In total, from the eight cSCC, seven responded completely to augmented, time protracted and adjuvant-enhanced immunocryosurgery cycles. From these initially responders six tumor sites remained relapse-free for at least 12 months after treatment.
To date, immunocryosurgery has been successfully applied to treat BCC,5 Bowen's disease6 and lentigo maligna.7 Immunocryosurgery was not considered a treatment option for cSCC, as cryosurgery ranks third line, mainly palliative modality, and imiquimod is not suggested for cSCC.3 However, the lasting efficacy of immunocryosurgery in the case of BCC is indicative of a synergistic interaction of imiquimod and cryosurgery within the proposed timing scheme with an underlying activation of immunostimulatory mechanisms both at tissue and at systemic levels.8
Surgery is not generally contraindicated in the oldest old although due to co-existing frailty often a not fully guideline-concordant surgical treatment may be applied.9
Conclusively, in this age group minimally invasive procedures, immunocryosurgery-based schemes or immunocryosurgery-like combinations of topicals (imiquimod, tretinoin cream and 2% 5-fluoruracil solution) with cryosurgery10 offer the advantage of flexible approach to individualized treatment needs to improve feasibility and effectiveness with minimal treatment burden.
Conflict of interestsThe authors declare that they have no conflict of interest.
The authors wish to express their appreciation to Professor Petia Radeva for language editing.