The COVID-19 pandemic may have adversely affected the early diagnosis of skin cancer.
ObjectiveTo compare epidemiological, clinical and histopathological characteristics in patients undergoing cutaneous squamous cell carcinoma (SCC) surgery before and after the beginning of the pandemic.
Material & methodsWe conducted a cross-sectional study including two case series: (1) patients operated on for SCC in the year after the first state of alarm in Spain (15 March 2020), and (2) patients with SCC operated on in the previous year. Epidemiological, clinical and histopathological variables, tumour stage and risk grade were collected.
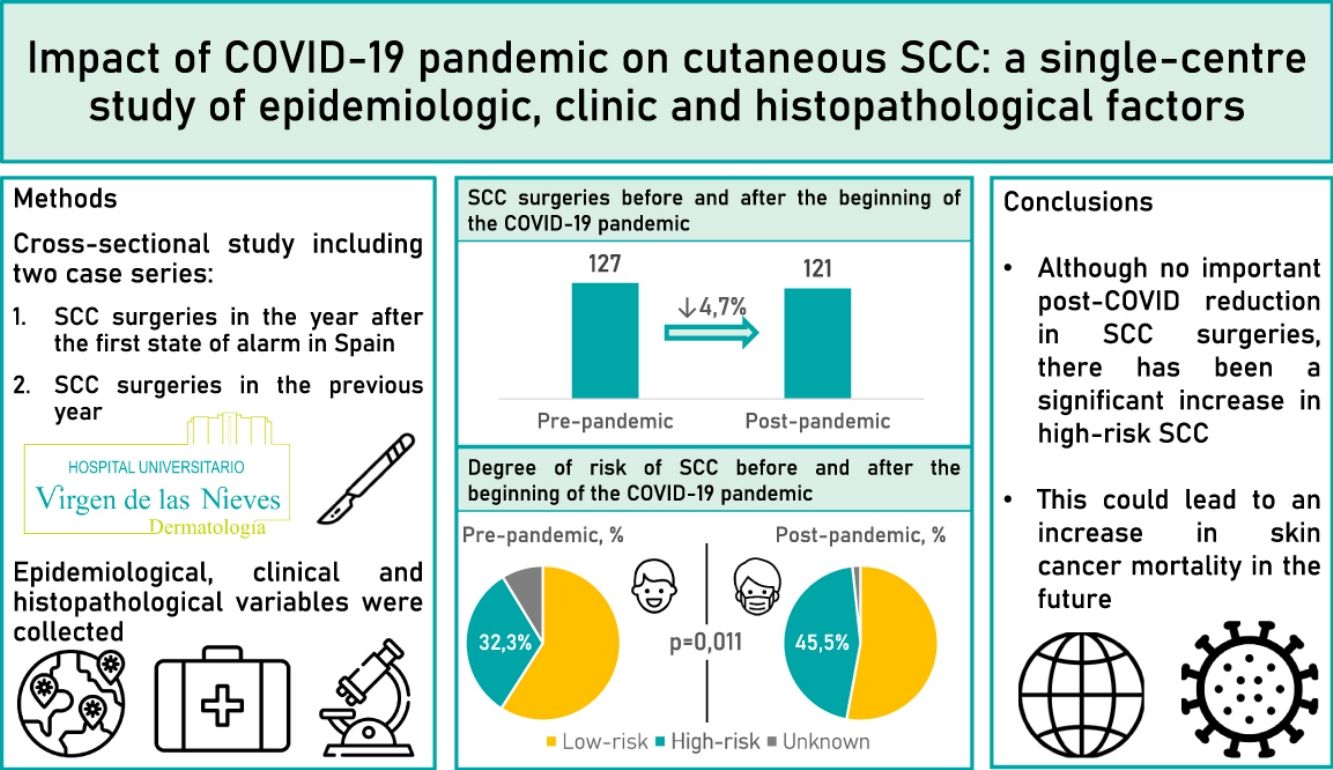
Results248 patients were included (127 undergoing surgery before the pandemic and 121 after the pandemic). After the beginning of the pandemic, the percentage of high-risk SCC significantly increased from 35.3% to 46.2% (p=0.011). However, no significant differences were found in thickness, perineural invasion or metastases.
ConclusionsAlthough there has not been a significant reduction in the number of SCC operated on after the pandemic, there has been a significant increase in high-risk SCC. All this could lead to an increase in skin cancer mortality in the future.
La pandemia de COVID-19 ha podido afectar negativamente el diagnóstico precoz del cáncer de piel.
ObjetivoComparar las características epidemiológicas, clínicas e histopatológicas en los pacientes intervenidos de carcinoma de células escamosas (CCE) cutáneo antes de la pandemia y después del inicio de la pandemia.
Material y métodosSe diseñó un estudio transversal que incluía 2 series de pacientes: 1) pacientes intervenidos de CCE el año posterior a la declaración del confinamiento general en España (15 de marzo de 2020), y 2) pacientes intervenidos de CCE el año previo. Se recogieron variables epidemiológicas, clínicas e histopatológicas, así como el estadio tumoral y el grado de riesgo.
ResultadosSe incluyeron 248 pacientes (127 intervenidos antes de la pandemia y 121 intervenidos después de la pandemia). Tras el inicio de la pandemia, el porcentaje de CCE de alto riesgo aumentó significativamente de 32,3 a 45,5% (p=0,011). No obstante, no se encontraron diferencias significativas en el grosor tumoral, la invasión perineural o la presencia de metástasis.
ConclusionesAunque no se produjo una reducción significativa en el número de CCE intervenidos después de la pandemia, ha habido un incremento significativo en los CCE de alto riesgo. Todo ello puede conllevar un incremento en la mortalidad por cáncer de piel en el futuro.
One of the main healthcare activities in the practice of dermatology is the diagnosis and treatment of malignant tumours, like squamous cell carcinoma (SCC), which has seen increased incidence.1 There are certain factors that are related to the prognosis of SCC and whose presence defines SCC as high-risk SCC. These include clinical factors like size (>2cm), location (lip, ear, temple), recurrence, neurological symptoms and immunosuppression. Histological high-risk factors encompass thickness (>6mm), invasion beyond subcutaneous fat, poor cell differentiation, perineural invasion (>0.1mm nerve fibres), lymphovascular invasion and high-risk histological types (adenosquamous, acantholytic, desmoplastic, spindle cell).2 Delays in SCC surgery, over 18 months, associate with thicker, invasive tumours.3
In December 2019, severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2) emerged in Wuhan, China, leading to over 762 million cases and more than 6.9 million deaths worldwide.4 COVID-19 pandemic has forced lifestyle changes in patients through confinements and quarantines in the most affected countries.5,6 In dermatology, teleconsultation has become widespread during the worst moments of the pandemic. It has allowed non-face-to-face care to be maintained.7,8 Being a very useful tool for the early detection of skin cancer, it does not provide a complete body examination and there is a risk of under-diagnosis.9–11 The real effects of COVID-19 pandemic on early skin cancer diagnosis remain unclear. This study aims to compare epidemiological, clinical and histopathological aspects of patients who had SCC surgery one year before Spain lockdown declaration with those who underwent surgery during the same period after lockdown.
Patients and methodsStudy designAn observational, single-centre, cross-sectional study was conducted.
Study populationOur study population comprised those patients who underwent surgery for SCC in the dermatology department of the Hospital Universitario Virgen de las Nieves between 15 March 2020 and 15 March 2021. These patients were compared with those who were surgically treated for SCC during the same period between 2019 and 2020.
Inclusion criteria were: patients over 18 years of age diagnosed with SCC through histological study in the established study periods, complete staging through clinical assessment and/or imaging tests according to the 2018 AJCC staging system.12 Patients who did not meet these criteria were excluded from the study.
Data sourceData collection was based on the medical records of each patient and the anatomopathological reports of tumour biopsies.
VariablesData were collected on age, sex, immunosuppression status, lesion location, time of diagnosis (pre-pandemic onset, post-pandemic onset), histopathological features of SCC (tumour thickness, tumour invasion, ulceration, histological type, degree of cell differentiation, lymphovascular invasion and perineural invasion), presence of distant metastases, TNM staging, tumour staging according to the 2018 AJCC staging system12 and SCC degree of risk. Regarding the degree of risk, high-risk SCC were considered to be those in which a risk characteristic was present.2 Clinical high-risk factors include horizontal size greater than 2cm, location on the lower lip, ear or temple, recurrent tumour, diameter growth rate greater than 4mm per month,13 neurological symptoms (pain and paraesthesia, pruritus or motor paralysis) and immunosuppression (transplantation, haematological malignancy, chronic immunosuppressive therapy, HIV positive). High-risk factors related to tumour histology are tumour thickness greater than 6mm, tumour invasion beyond the subcutaneous fat, poor degree of cell differentiation, perineural invasion (nerve fibres greater than 0.1mm in diameter), lymphovascular invasion and high-risk histological variants (adenosquamous, acantholytic, desmoplastic and spindle cell).
The number of tumours operated on and the percentage of high-risk tumours were stratified on a quarterly basis to determine their distribution over the study periods. Each study period was divided into four: 15 March–14 June, 15 June–14 September, 15 September–14 December and 15 December–14 March.
The activity in the consulting room and operating theatre of our department was not interrupted during the pandemic.
Statistical analysisA descriptive analysis of the data was performed. We obtained means and standard deviations for quantitative variables and percentages for quantitative variables, in order to show the characteristics of the sample collected. Contingency tables were used for the comparison of quantitative variables and the chi-square test or Fisher's exact test was used when necessary. Differences with a p-value<0.05 were accepted as statistically significant. IBM SPSS version 28 software was used for the statistical analysis.
Ethical aspectsThis study was performed by collecting data from medical and anatomopathological reports, without interventions that could pose any risk to the patients. This study was approved by the Ethics Committee of the Hospital Universitario Virgen de las Nieves (protocol code 1267-N-22).
ResultsWe studied 248 patients: 127 pre-pandemic and 121 post-pandemic, a 4.7% decrease (Table 1). Average age shifted from 79.3 to 76.5 post-pandemic (p=0.046). Males rose from 55.9% to 68.6% post-pandemic (p=0.04). Immunocompromised patients were 12.6% pre-pandemic and 18.2% post-pandemic.
Epidemiological factors, clinical and histopathological characteristics, staging and risk status of patients undergoing SCC surgery before and after the pandemic.
| Pre-pandemic, N (%) | Pandemic, N (%) | p | |
|---|---|---|---|
| Agea | 79.3 (10.6) | 76.5 (10.8) | 0.046 |
| Sex | 0.04 | ||
| Male | 71 (55.9) | 83 (68.6) | |
| Female | 56 (44.1) | 38 (31.4) | |
| Immunosuppression | 0.22 | ||
| Yes | 16 (12.6) | 22 (18.2) | |
| No | 111 (87.4) | 99 (81.8) | |
| Location | 0.09 | ||
| Face | 51 (40.2) | 39 (32.2) | |
| Temple | 8 (6.3) | 8 (6.6) | |
| Scalp | 22 (17.3) | 19 (15.7) | |
| Ear | 14 (11.0) | 12 (9.9) | |
| Lower lip | 3 (2.4) | 6 (5.0) | |
| Trunk | 8 (6.3) | 9 (7.4) | |
| Upper limb | 7 (5.5) | 21 (17.4) | |
| Lower limb | 14 (11.0) | 7 (5.8) | |
| Tumour thicknessa | |||
| Total | 3.01 (3.16) | 3.27 (3.18) | 0.49 |
| Invasivesb | 4.05 (3.02) | 4.27 (2.99) | 0.61 |
| Tumour invasion | 0.34 | ||
| In situ | 30 (25.6) | 27 (22.3) | |
| Invasive | 87 (74.4) | 92 (76.0) | |
| Unknown | 0 (0.0) | 2 (1.7) | |
| Ulceration | 0.37 | ||
| Yes | 38 (29.9) | 30 (24.8) | |
| No | 89 (70.1) | 91 (75.2) | |
| Histological type of risk | 0.43 | ||
| Yes | 3 (2.4) | 5 (4.1) | |
| No | 124 (97.6) | 116 (95.9) | |
| Differentiationc | 0.48 | ||
| Well | 31 (32.0) | 30 (31.6) | |
| Moderate | 43 (44.3) | 49 (51.6) | |
| Poor | 18 (18.5) | 15 (15.8) | |
| Unknown | 5 (5.2) | 1 (1.0) | |
| Linfovascular invasion | 0.13 | ||
| Yes | 0 (0.0) | 1 (0.8) | |
| No | 116 (91.3) | 116 (95.9) | |
| Unknown | 11 (8.7) | 4 (3.3) | |
| Perineural invasion | 0.95 | ||
| Yes | 4 (3.1) | 4 (3.3) | |
| No | 110 (86.6) | 114 (94.2) | |
| Unknown | 13 (10.2) | 3 (2.5) | |
| Distant metastases | 0.31 | ||
| Yes | 0 (0.0) | 1 (0.8) | |
| No | 117 (92.1) | 118 (97.5) | |
| Unknown | 10 (7.9) | 2 (1.7) | |
| T | |||
| IS | 30 (23.6) | 27 (22.3) | 0.68 |
| 1 | 55 (43.3) | 53 (43.8) | 0.66 |
| 2 | 14 (12.0) | 9 (7.4) | 0.25 |
| 3 | 18 (14.2) | 29 (24.0) | 0.08 |
| 4 | 0 (0.0) | 1 (0.8) | 0.32 |
| Unknown | 10 (7.9) | 2 (1.7) | |
| N | 0.32 | ||
| 0 | 117 (92.1) | 118 (97.5) | |
| + | 0 (0.0) | 1 (0.8) | |
| Unknown | 10 (7.9) | 2 (1.7) | |
| M | 0.32 | ||
| 0 | 117 (92.1) | 118 (97.5) | |
| 1 | 0 (0.0) | 1 (0.8) | |
| Unknown | 10 (7.9) | 2 (1.7) | |
| Stage | |||
| In situ | 30 (23.6) | 27 (22.3) | 0.68 |
| I | 56 (44.1) | 54 (44.6) | 0.70 |
| II | 13 (10.2) | 8 (6.6) | 0.24 |
| III | 18 (14.2) | 29 (24.0) | 0.08 |
| IV | 0 (0.0) | 1 (0.8) | 0.32 |
| Unknown | 10 (7.9) | 2 (1.7) | |
| High-risk SCC | 0.011 | ||
| Yes | 41 (32.3) | 55 (45.4) | |
| No | 75 (59.1) | 64 (52.9) | |
| Unknown | 11 (8.7) | 2 (1.7) | |
| Total | 127 (100) | 121 (100) | |
Face and scalp were common sites, with the upper limb increasing post-pandemic. Thickness averaged 3.01mm pre-pandemic and 3.27mm post-pandemic (p=0.49). Excluding in situ SCC, it was 4.05mm pre-pandemic and 4.27mm post-pandemic (p=0.61). Invasive SCC were 74.4% pre-pandemic and 76.0% post-pandemic (p=0.34). No differences in ulceration, histological type, cell differentiation, perineural invasion or lymphovascular invasion were noted. No significant difference in distant metastases was found.
Regarding tumour staging T1 was 43.3% pre-pandemic and 43.8% post-pandemic. T2 and T3 were 12.0% and 14.2% pre-pandemic, changing to 7.4% and 24.0% post-pandemic, respectively. One post-pandemic T4 tumour was noted. Only one SCC N+ was found post-pandemic. Stage I was 44.1% pre-pandemic and 44.6% post-pandemic. Stages II and III were 10.2% and 14.2% pre-pandemic and 6.6% and 24.0% post-pandemic, respectively. One stage IV SCC post-pandemic was identified.
Regarding risk, high-risk SCC rise from 32.3% pre-pandemic to 45.4% post-pandemic (p=0.011).
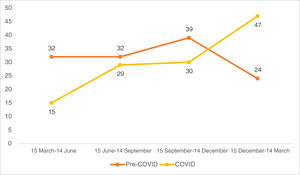
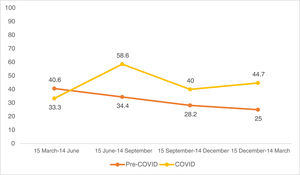
Looking at the quarterly distribution of surgeries before the COVID-19 pandemic: 32 SCC surgeries between 15 March and 14 June, 32 between 15 June and 14 September, 39 between 15 September and 14 December and 24 between 15 December and 14 March (Fig. 1). The percentage of high-risk SCC during these periods was 40.6%, 34.4%, 28.2% and 25.0%, respectively (Fig. 2). After Spain general lockdown declaration, 15 SCC surgeries occurred between 15 March and 14 June, 29 between 15 June and 14 September, 30 between 15 September and 14 December and 47 between 15 December and 14 March. The percentage of high-risk tumours were 33.3%, 58.6%, 40.0% and 44.7%, respectively.
DiscussionDuring lockdown, medical visits and surgical activity were reduced to the bare minimum. In addition, fear of infection caused the general population to delay or even avoid seeking medical care.14 Thus, the SARS-CoV-2 pandemic has had a significant impact on both the diagnosis of new tumours and their prognosis.15–17 In our sample, we found a 4.7% reduction in the number of SCC intervened after lockdown declaration. Most studies performed so far have focused on the first months after lockdown declaration and show a substantial reduction in the number of SCC interventions.14,18 However, our study includes patients treated up to one year after the lockdown declaration in Spain. Additionally, unlike in other centres, the activity in the consulting room and operating theatre of our department was not interrupted at any time, with teleconsultation being promoted.
According to a recent study, during the months of confinement there was a significant decrease in diagnosed keratinocytic tumours compared to previous years. However, after the end of confinement there was an increase in the diagnosis of keratinocytic tumours.19 Another study conducted in Italy reports that more skin cancer surgeries were performed during the months of May to November 2020 than during the same period in 2019.20 In our case, when breaking down the data quarterly, SCC surgeries decreased from 32 to 15 in the first quarter. The general lockdown period in Spain took place between 15 March and 21 June 2020. The vast majority of this period was collected in the first quarter of the COVID study period. Later, the numbers rose, with post-pandemic surgeries between 15 December and March 14 nearly doubling compared to the same pre-pandemic period. For this reason, we believe that in our sample no major differences were observed in the number of SCC surgeries before lockdown compared to those performed afterwards.
In a study carried out in our centre, we did observe differences in the reduction of melanoma surgery after the pandemic.21 The fact that we did not find large differences in the SCC surgeries could be due to the fact that these are more visually aggressive tumours. SCC are associated with greater episodes of bleeding and ulceration and usually appear in exposed areas, whereas melanomas are often flat lesions that tend to go unnoticed for longer.22,23
Likewise, in our study we found a higher percentage of high-risk SCC after the pandemic. Several studies agree with this finding, although, as mentioned above, they focus on the first months of the pandemic. In a multicentre study carried out in Spain and focusing on the months of lockdown, the authors observed that the same number of large SCC (>4cm) underwent surgery during this time as in the 3 months prior to pandemic confinement.14 However, these large SCC accounted for a higher proportion of the tumours treated after lockdown, as the number of operations on tumours smaller than 4cm was reduced. This may be due to the recommendations made by different organizations during the months when hospitals were most overcrowded. They recommended prioritizing more advanced tumours over lower-risk lesions for which intervention could be delayed.17,24,25
In our study, including patients who underwent surgery beyond the first few months after confinement, we found a higher percentage of high-risk tumours after the pandemic, especially in the periods that followed the general lockdown. Quarterly stratification in our post-COVID sample suggests a delayed diagnosis. Patients who did not seek consultation in the initial months of the pandemic did so in subsequent months, as mentioned earlier. In this way, it is possible that treatment delay of low-risk lesions may have led to the appearance of any feature that transforms these into high-risk tumours.26,27 Alternative explanations for the increase in high-risk tumours, such as the involvement of the virus itself or agents used in the treatment and prophylaxis of COVID-19 infection, there is currently insufficient evidence to support a significant impact of these factors on the rise in high-risk tumours.
Furthermore, we have not found significant differences in those variables that determine the degree of risk of SCC. This may be fundamentally due to a lack of statistical power, and makes it necessary to carry out more studies like ours to find out the real effects of the pandemic on SCC.
In the aforementioned study carried out at our centre, significant differences were found in terms of thickness or tumour stage of melanomas.21 The fact that no such clear differences were found in patients with SCC in the risk variables may be due to a lower average growth rate of SCC compared to melanomas.13
With all these facts, a worse prognosis can be expected for patients with SCC diagnosed after general confinements caused by the pandemic.
The main strength of our study is that we have described the effects of the pandemic on SCC up to one year after the declaration of general lockdown. Nevertheless, we are aware that the study is subject to several limitations. Firstly, being a single-centre study, the sample is limited and this could make it difficult to achieve statistical significance in some of the variables studied. On the other hand, as it is a retrospective study, it is possible that there is information not collected on the patients seen during both periods. Furthermore, given that this is a cross-sectional study, it is difficult to establish causal associations. Still, it is a good starting point for future prospective studies with longer follow-up times.
ConclusionsWe observed no significant differences in the number of SCC surgeries after lockdown compared to the same period before lockdown. Nevertheless, we did find a significant increase in the number of high-risk SCC after the pandemic. Delayed diagnosis and treatment of SCC during lockdown may have led to an increase in the number of high-risk lesions afterwards. However, further studies are needed to understand the true effects of the pandemic on skin cancer.
Conflict of interestsThe authors declare that they have no conflict of interest.
This paper is part of the PhD project of Pablo Díaz-Calvillo.