The management of Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) is a real therapeutic challenge. While withdrawal of the offending drug is the mainstay of therapy, there is still much controversy surrounding systemic treatment and wound care. This is a case report of severe toxic epidermal necrolysis requiring multimodal management, including admission to a burn unit and treatment with human amniotic membrane (HAM) grafting, resulting in complete remission.
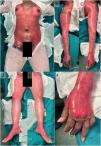
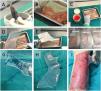
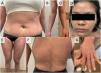
A 27-year-old woman with no relevant past medical history was referred from her primary care center for skin lesion assessment. She reported that the eruption began 7 days earlier as red-purple macules and plaques, predominantly acral, which were initially treated as mild erythema multiforme with topical corticosteroids. However, the patient presented with progression of the lesions. On examination (Fig. 1), there was epidermal sloughing involving approximately 85% of the body surface area, with sparing of the face and portions of the neck and genital region. Suspecting TEN, she reported taking cefuroxime for cystitis 8 days prior to symptom onset. She was admitted to the burn unit, where she was started on fluid therapy, closely monitored organically and treated with antiseptic dressings. The SCORTEN scale was calculated with an admission score of 3 (heart rate≥120/min, body surface area involved≥10%, plasma urea≥60mg/dL) and the visual analog scale (VAS) score on admission was 10/10. Treatment with prednisone at a dose of 1mg/kg/day was also started, a skin biopsy was performed which confirmed the diagnosis of TEN. Viral involvement was ruled out by polymerase chain reaction (PCR) for respiratory viruses in the nasopharynx. The patient showed an overall favorable course after admission; however, some areas of skin with persistent sloughing refractory to antiseptic dressings remained (approximately 6% of the total body surface area). In these areas, HAM grafting was applied (Fig. 2), resulting in a very satisfactory response and complete epithelialization. The HAM was maintained for 7 days. The patient was discharged from hospital 15 days after symptom onset, with a VAS score of 1/10 at discharge. At the patient's follow-up 10 days after discharge (Fig. 3), no primary skin lesions were noted, except for post-inflammatory hyperpigmentation and small scarring areas in the gluteal region. In a subsequent telephone consultation 3 months after discharge, the patient reported being completely asymptomatic.
SJS and TEN are 2 rare and life-threatening conditions caused almost exclusively by adverse drug reactions. To date, there are only 5 articles in the literature with evidence-based guidelines for the management of SJS and TEN. In fact, the use of systemic immunosuppressants remains controversial.
Classically, the management of skin wounds in patients with TEN has involved periodic dressings with antiseptic dressings (e.g. Mepilex Ag®) or biosynthetic skin substitutes (e.g., Biobrane®).1
The HAM is a thin, semi-transparent membrane composed of 3 distinct layers: an inner epithelial layer, a thick intermediate basement membrane and an outer stromal layer.
The use of HAM for therapeutic purposes began in 1913 when Stern and Sabella described its use in burn patients. Since then, various groups have used it to treat corneal involvement in TEN2,3 and, more recently, it is beginning to be used routinely as a temporary biologic dressing in patients with burns or other severe skin involvement (as in the case of SJS and TEN), as it has been shown to provide growth factors, collagen and angiogenic factors that promote re-epithelialization, reduce pain with excellent tolerability and minimal immunogenicity.4
The HAM is obtained through altruistic donations from obstetric patients in previous deliveries, according to the standards of the Spanish Association of Tissue Banks. It is not commercially available. Membranes were obtained only from patients undergoing C-section to avoid contamination through the birth canal, and after extraction the placenta was washed with saline to remove any traces of blood and stored at 4°C. Afterwards, they were placed in a combined solution containing 50μg/mL amphotericin, 50μg/mL penicillin and 50μg/mL streptomycin for a maximum of 24h. In the laboratory, under a laminar flow chamber, the amniotic membrane was identified, and dissection was performed to separate the membrane from the rest of the placenta. The stromal part of the membrane was located, placed on the filter paper used as a support and cut out. The fragments were placed in labeled containers ready for application.5 The graft was applied directly to the affected area after prior cleansing with physiologic saline. Subsequently, it is compressed with usual dressings. The HAM maintenance time varies, ranging from 5 to 15 days. Various cures can be performed with amniotic membrane, depending on the defect.
With this case, we highlight that the HAM graft can be used in patients with SJS or TEN in appropriate situations.
Conflict of interestThe authors declare that they have no conflict of interest.