Dupilumab is a recombinant human IgG4 monoclonal antibody that inhibits interleukins (IL)-4 and IL-13 by specifically binding to the IL-4Rα subunit of their receptor complexes.1 IL-4 has been detected in biopsies of chronic spontaneous urticaria (CSU),2 and the efficacy of dupilumab has been described in CSU1,3–5 and in inducible chronic urticaria (ICU) (e.g., cold urticaria,6 cholinergic,7 or solar urticaria8). Below, we describe 2 cases, one with CSU and the other with ICU in the form of delayed pressure urticaria (DPU), refractory to omalizumab, with an excellent response to dupilumab.
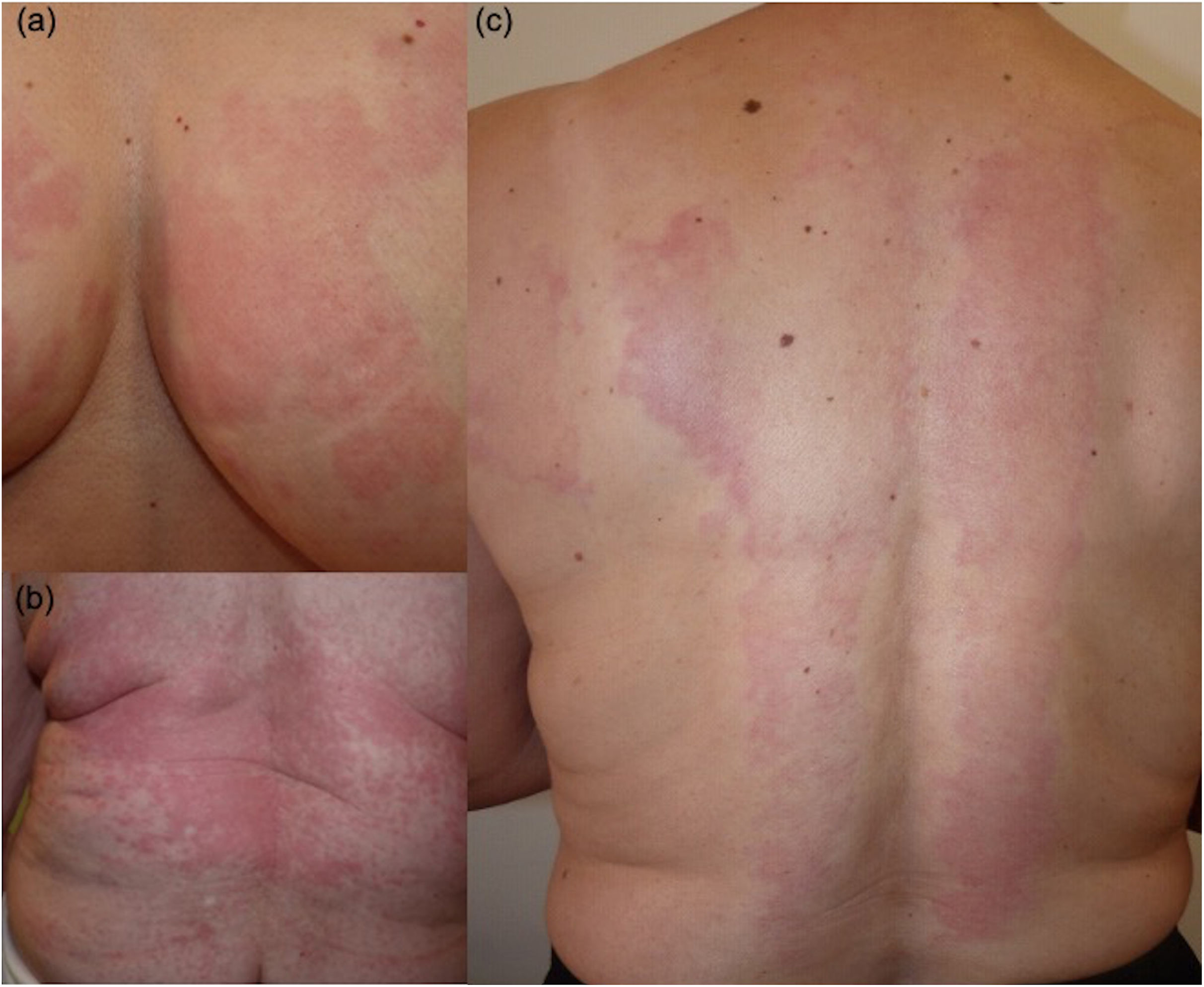
Case #1. In 2012, a 67-year-old woman with a past medical history of diabetes, hypertension, dyslipidemia, and autoimmune thyroiditis, with CSU and ICU of the DPU type (Fig. 1a–c) refractory to antihistamines and cyclosporine was treated with omalizumab (300mg every 15 days) with a rapid resolution of symptoms. In 2018, after remaining asymptomatic for several years, omalizumab was discontinued, and a relapse was reported shortly thereafter, linked to stressful events. When she failed to respond to intensified treatment (omalizumab 600mg every 3–4 weeks) along with antihistamines (×4), other systemic drugs (cyclosporine, dapsone, methotrexate, montelukast, and azathioprine, along with weekly dexamethasone pulses) were introduced without improvement. The histopathological study showed dermal interstitial infiltrates of neutrophils and eosinophils, with no vascular damage. The suspended weight test was positive.
In February 2022, the patient had a Urticaria Activity Score (UAS)7>20, with a severe impact on her quality of life, requiring daily oral corticosteroids. At that time, treatment with dupilumab (600mg on day 0 followed by 300mg biweekly) was started, with initial improvement of itching, although oral corticosteroids were still required. After 2 months, she experienced an exacerbation attributed to COVID-19 (with DPU due to prolonged bed rest). The patient began responding after 3 months and the corticosteroid was gradually down titrated until it was completely discontinued by month 5. IgE levels dropped from 6KU/L down to 4KU/L 3 months after starting treatment. Afterwards, she remained asymptomatic without corticosteroids for the following 11 months (16 months from the start of treatment).
Case #2: A 59-year-old female physician, with a past medical history of hay fever and autoimmune thyroiditis, developed 2 episodes of CSU between 2011 and 2014 which went on for several months each. In January 2020, she presented with CSU again, along with angioedema and DPU. The histopathological study showed dermal interstitial inflammation with eosinophils, with no vasculitis.
She was put on antihistamines (×4) and prednisone, without improvement, and required sick leave due to sleep deprivation. Omalizumab (300mg every 4 weeks) was then introduced, with good initial control (IgE increased from 19.9KU/L up to 65.7KU/L 1 month after starting therapy), but due to stress from the pandemic (COVID-19), in March 2020, disease control deteriorated, and omalizumab was up titrated (600mg monthly), with the addition of prednisone (5–10mg) for 2 years due to the persistence of hives (UAS: 24–42) (Fig. 2a), angioedema (Fig. 2b), and deterioration in quality of life (DLQI=30). The suspended weight test was repeatedly positive (Fig. 2c).
Cyclosporine was ruled out because the patient regularly attended to COVID-19 patients. Therefore, treatment with dupilumab (600mg on day 0 and 300mg every 2 weeks) was started, although lesions persisted during the first 4 months. However, from month 5, she started experiencing progressive improvement, with complete resolution of the hives and angioedema by month 7. IgE levels modestly increased from 42.4KU/L (before the first dose of dupilumab) up to 46.5KU/L (1 month after starting). She remained asymptomatic without corticosteroids at the 12-month follow-up (19 months since the start of treatment).
After discontinuing omalizumab due to good control, more than 90% of patients respond to retreatment.9 The association with autoimmune disease (diabetes mellitus or autoimmune thyroiditis), low IgE levels (especially in the first case), the presence of angioedema (in case #2), DPU itself (a particularly refractory variant of ICU), and stress may have contributed to an unfavorable response to omalizumab (with refractoriness upon reintroduction in case #1 and loss of efficacy in case #2). It cannot be determined whether an intensified regimen of dupilumab would have shortened the response time in these cases, as there are no precedents of DPU treated with this drug.
Dupilumab emerges as a potential alternative for treating omalizumab-refractory CSU and ICU. In our experience, the response may not occur immediately but rather after 3–4 months from the start of treatment.
Conflicts of interestNone declared.