Graying is a natural process, characterized by the loss of pigment in the hair, and is considered a sign of aging in society; its onset and progression is closely correlated with age and occurs to varying degrees in all individuals, regardless of gender or race. However, premature graying has a psychological and social impact, affecting patients’ self-esteem. The exact etiopathogenesis is still unknown, and it has been associated with multiple factors such as: oxidative stress, ultraviolet radiation, genetic factors, and lifestyle factors such as smoking, nutritional and emotional factors. All of this causes a decrease in the production of melanin by the melanocytes of the hair bulb. Despite the extensive molecular research being carried out to understand the pathogenesis of graying, there is a shortage of effective, evidence-based treatment options. Today, graying has become a cultural phenomenon, where many people opt for cosmetic treatments to cover gray hair.
Hair graying—also known as gray hair, white hair, or achromotrichia—is considered one of the earliest normal signs of aging. It usually appears around 34 years of age in Caucasians and 43 years in individuals of African descent.1 However, it is defined as premature graying (PG) when at least 5 gray hairs appear before the age of 20 years in Europeans, 25 gray hairs in Asians, and before 30 years of age in Africans.2
Hair symbolizes well-being and health and plays an important role in social communication, self-perception, self-esteem, and body Image.3 Recently, the treatment of premature graying has been increasingly studied due to its psychological consequences and its possible association with metabolic diseases.4,5
Hair follicle melanogenesisHair follicle melanocytes are categorized into subpopulations according to their function, degree of differentiation, and location.
The first location is in the hair bulb during the anagen phase, in the upper region of the dermal papilla. Bulbar melanocytes are the only cells that contribute to hair pigmentation. They express active tyrosinase and dihydroxyphenylalanine (DOPA) and are considered part of the hair follicle pigmentary unit, along with keratinocytes.6
Melanogenesis occurs within specialized lysosomes called melanosomes, which are transferred to keratinocytes via dendrites and filopodia. This process occurs exclusively during the anagen phase, ceases during catagen, and remains inactive during telogen. Melanosomes transfer melanin primarily to the hair cortex, to a lesser extent to the medulla, and only occasionally to the cuticle.7
Unlike epidermal melanocytes, the hair bulb contains approximately 1 melanocyte for every 5 keratinocytes, and hair follicle melanocytes have longer and more numerous dendrites.8
The second melanocyte location within the hair follicle is the bulge area. These melanocytes are immature and inactive, do not express melanogenic enzymes such as tyrosinase or tyrosinase-related protein 1 (TRP-1), and serve as precursor cells. They proliferate, mature, and migrate to the bulb during anagen.9
Melanocytes have also been identified in the infundibulum, similar to epidermal melanocytes, and in sebaceous glands6 (Table 1).
Key signaling pathways involved in melanogenesis.
| Signaling pathway | |
|---|---|
| Wnt/β-catenin pathway | Increases transcription of the melanocyte-inducing transcription factor in melanocyte stem cells.• Increases endothelin receptor type B signaling• Promotes migration, proliferation, differentiation, and melanogenesis |
| MC1R | • Melanogenesis and melanosome transfer• Repair of UV radiation–induced DNA damage |
| KIT/SCF | Regulation of melanin production |
| EDN/EDNRB | Melanocyte proliferation and melanogenesis |
| PI3K/AKT | • Extracellular release of melanin; prevention of oxidative stress, DNA damage, and reduced cell survival |
| TGF-β | Main regulator of melanocyte stem cells, causing cell cycle arrest and downregulation of MITF |
| MITF | Differentiation of melanocyte stem cells.• Positive regulation of pigmentation• Positive regulation of melanosomes• Facilitates melanocyte proliferation• Antiapoptotic• Mitigates DNA damage |
The hair follicle pigmentary unit reaches its maximum melanin production during adolescence, followed by a gradual decline over the years.
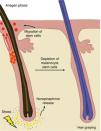
Repigmentation and reconstruction of the pigmentary unit are lost with each hair cycle and can only be achieved for 7 to 15 cycles, corresponding to approximately 40–45 years of age, resulting in reduced pigment production. Another contributing factor is the prolongation of the telogen phase that occurs with aging10 (Fig. 1).
Pigment loss in the hair shaft is associated with reduced melanin content and a decrease in bulbar melanocytes. When hair becomes gray, few melanocytes remain, but they still express tyrosinase and continue melanin transfer to keratinocytes. In contrast, white hair lacks melanocytes in the bulb altogether.1
EtiopathogenesisThe exact etiopathogenesis of premature graying has not yet been fully elucidated. It may be associated with autosomal genetic disorders, premature aging syndromes such as progeria and pangeria, oxidative stress resulting from ultraviolet radiation, pollution, and emotional factors, as well as inflammatory causes. Several studies have also reported associations with deficiencies of vitamin B12, vitamin D, iron, calcium, ferritin, copper, and zinc, as well as with thyroid hormone deficiency and drugs that reduce melanogenesis.2
Oxidative stressOxidative stress plays a significant role in melanogenesis. Melanin synthesis generates hydrogen peroxide (H2O2), superoxide (O2−), and hydroxyl free radicals. Compared with keratinocytes, melanocytes are more vulnerable to oxidative stress.11
Defense mechanisms against oxidative stress include antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). However, their activity decreases with aging, creating an imbalance between oxidative damage and repair mechanisms.12
Excessive accumulation of reactive oxygen species (ROS) within hair follicle melanocytes leads to cellular damage through lipid peroxidation, DNA strand breaks, mutations, and protein and enzyme denaturation. Damage to the cell membrane generates arachidonic acid, followed by the formation of aldehydes such as malondialdehyde (MDA) and 4-hydroxynonenal. MDA crosses the cell membrane into the extracellular compartment; therefore, its serum concentration represents a sensitive marker of oxidative stress. Collectively, this damage results in decreased melanocyte pigment-forming capacity and may even induce apoptosis through activation of inflammatory pathways such as the MAPK and p53 pathways.13
Saxena et al. evaluated serum levels of MDA, reduced glutathione, and SOD in patients with premature graying and found significantly higher MDA levels vs controls. SOD levels were also lower in premature graying cases, reinforcing the role of oxidative stress in PG.14
Genetic factorsNumerous genes and signaling pathways involved in premature graying have been identified. Bian et al. reported reduced expression of genes responsible for melanin synthesis in the hair follicle—such as TYR, melan-A (MLANA), premelanosome protein (PMEL), TYRP1, SLC45A2, KIT, G protein–coupled receptor 143 (GPR143), and OCA2—in patients with premature graying.15
UV radiationUV radiation plays an important role in hair graying by stimulating the production of free radicals. UVA radiation can cause hair color changes by penetrating deeply into the cortex and inducing biochemical damage, whereas UVB radiation leads to protein depletion and structural damage to the cuticle.16,17
Tobacco useSmoking is associated with hair graying through increased free radical production. Shin et al. found a statistically significant association between smoking and premature graying, consistent with former studies.18,19
StressThe mechanism linking graying and stress is not yet fully understood.
Animal studies suggest that stress can accelerate hair graying. Sharma et al. evaluated stress levels in patients with PG using the Perceived Stress Scale (PSS) and found significantly higher stress scores in patients with PG vs controls.20
Zhang et al. demonstrated that acute stress can cause irreversible depletion of somatic stem cells via activation of the sympathetic nervous system, resulting in permanent tissue damage and hair graying.21
Hair follicles are innervated by sympathetic nerves; under stress conditions, they release norepinephrine, inducing proliferation of melanocyte stem cells that differentiate and migrate out of the bulge.10 This leads to depletion of melanocyte stem cells without replacement. Without melanocytic cells to provide pigment during the next anagen phase, hair appears white10,22 (Fig. 2).
Various stressors—including nociception-induced stress, chronic stress, and restraint stress—have been shown to cause melanocyte stem cell loss and hair graying through immunological mechanisms, corticosterone, and norepinephrine.22,23
Melanocyte stem cells express β2-adrenergic receptors, which respond to norepinephrine. Loss of this receptor completely blocks stress-induced hair graying.23 Other stress-related mediators include calcitonin gene-related peptide, which maintains immune privilege and induces catagen while promoting melanogenesis; substance P, which downregulates growth, induces apoptosis, and increases free radical production; and vasoactive intestinal peptide, which prevents follicular collapse.24,25
Adipose tissue surrounding hair follicles is rich in growth factors that regulate stem cell activation and regeneration and repress Wnt signaling.22,24
Nutritional factorsHair may exhibit reversible hypopigmentation due to nutritional deficiencies. Daulatabad et al. found lower vitamin B12 levels in patients with PG vs controls, with greater deficiency associated with increased severity. They also found lower folic acid levels but no differences in biotin levels.26
Sharma et al. reported lower serum calcium and ferritin, lower HDL cholesterol, and higher LDL cholesterol levels in patients with PG vs controls.20 Premature graying has been linked to early cardiovascular disease, and dyslipidemia may serve as a connecting factor.5
Furthermore, lower vitamin D levels have been reported in patients with PG, although without statistically significant associations.20,27 Regarding copper, lower levels have been observed in PG patients vs controls, but without statistical significance.28,29
ComorbiditiesSeveral studies have reported a higher prevalence of atopic diathesis in patients with PG.20,26,30 Das et al. conducted a study in patients with PG and found higher levels of blood pressure, glucose, insulin, C-reactive protein, and HOMA index, as well as elevated IL-6 levels, compared with controls. These findings suggest an increased cardiovascular risk, leading the authors to propose screening for cardiovascular risk factors in patients with PG.5
Chandran et al. measured thyroid hormone levels in patients with PG and found higher anti-thyroid peroxidase (TPO) antibody levels vs controls; however, no differences were observed in T3, T4, or TSH levels.31
An increased risk of osteopenia—up to fourfold higher—has been reported in patients with PG vs individuals without gray hair.32 Nevertheless, more recent studies failed to confirm this association.33
In cases of PG, a laboratory work-up may be necessary, including iron studies, vitamin B12, folic acid, 25-hydroxyvitamin D, thyroid profile, and antithyroid antibodies.31–33
Clinical featuresGray hair primarily results from an optical phenomenon. The pale yellow keratin of non-pigmented hair appears white due to light refraction or reflection.34 Compared with pigmented hair, gray hair is thicker, stiffer, and more difficult to manage, grows faster, and is more susceptible to UV radiation damage, requiring greater protection.35 Alterations in hair fiber make it more difficult to maintain artificial color, leading many individuals to undergo repeated dyeing procedures, which may result in toxicity from artificial hair dyes.36
There are no sex-related differences in the prevalence of premature graying.37
In women, gray hair usually begins along the hairline, whereas in men it typically starts in the temporal region and sideburns. Graying first affects the scalp, followed by the facial area, and finally body hair.
ClassificationThere is no standardized scale for classifying graying. Several have been proposed; one of the most widely used is the Hair Whitening Score (HWS), which is based on the percentage of affected hair38:
- •
Trace: <25%
- •
Mild: 25–50%
- •
Moderate: 50–75%
- •
Marked: 75–100%
- •
Complete: 100%
It is necessary to distinguish graying from other conditions that cause localized or diffuse hair hypopigmentation (Table 2).
Differential diagnosis of premature graying.
| Disease | Characteristics |
|---|---|
| Localized causes of gray/white hair | |
| Vitiligo | Achromic patches that may be accompanied by poliosis |
| Piebaldism | Congenital pigmentary disorder characterized by areas of leukoderma and poliosis, secondary to KIT gene mutation |
| Waardenburg syndrome | Autosomal dominant disorder characterized by sensorineural deafness, pigmentary abnormalities, and iris heterochromia |
| Diffuse gray/white hair causes | |
| Oculocutaneous albinism | Group of autosomal recessive disorders characterized by inability of melanocytes to produce pigment in skin, hair, and eyes |
| Chediak–Higashi syndrome | Partial oculocutaneous albinism, immunodeficiency, and recurrent bacterial infections |
| Hermansky–Pudlak syndrome | Oculocutaneous albinism, platelet dysfunction, and lysosomal storage defects |
| Griscelli syndrome | Partial albinism, photosensitivity, silvery-gray hair, and neurological disorders |
| Menkes syndrome | Copper metabolism disorder, hypopigmented, sparse, brittle hair with steel-wool appearance, associated with hair shaft abnormalities |
| Elejalde syndrome | Silvery hair, central nervous system dysfunction including seizures, hypotonia, and intellectual disability |
| Sudden graying | Alopecia areata, telogen effluvium, and vitiligo |
There are reports of abrupt overnight graying, known as sudden canities or Marie Antoinette syndrome, which is related to diffuse alopecia areata. In this condition, pigmented hair is preferentially shed, leaving only hypopigmented hair.39,40
TreatmentDespite extensive research into the pathophysiology of graying, no satisfactory treatments currently exist (Table 3). Due to high patient demand, vitamin supplements, antioxidants, and minerals such as biotin, zinc, copper, selenium, and calcium pantothenate are frequently used, although their efficacy has not been demonstrated. If deficiencies of vitamin B12, folic acid, vitamin D, or thyroid hormone abnormalities are detected, specific treatment is recommended.
Calcium pantothenate and para-aminobenzoic acid (PABA) have been used, showing temporary hair repigmentation. Pasricha et al. reported hair repigmentation in 2 patients with PG on 200mg of calcium pantothenate daily.41 In a subsequent study using the same dose combined with plucking of gray hair, a reduction in the number of gray hairs was observed at a 3-year follow-up.42
Conversely, a prospective study evaluating 100mg of calcium pantothenate+200mg of PABA daily in 27 individuals with age-related graying and 6 with PG found that 6% (all with age-related graying) showed marked repigmentation and 21% showed slight improvement after 8 months. However, hair returned to gray after discontinuation of supplementation.34 No recent studies support the use of calcium pantothenate or PABA.
Topical treatmentPalmitoyl tetrapeptide-20 and melitane are biomimetic peptides that act as agonists of α-melanocyte-stimulating hormone (α-MSH) and, together with its melanocortin-1 receptor (MC1R), regulate pigment production in hair. Palmitoyl tetrapeptide-20 has been studied in vitro and in 15 men with premature graying, inducing hair pigmentation after 3 months.44 The use of melitane plus oral supplements has been reported in a 14-year-old woman, with favorable results observed after 6 months.45
Latanoprost has been studied in animal models, demonstrating increased follicular melanogenesis and hair growth.46
Dyes and colorantsMost patients resort to natural or artificial dyes. Natural dyes contain ingredients such as henna (Lawsonia alba), Indian gooseberry (Emblica officinalis), and false daisy (Eclipta alba), and are generally less irritating and allergenic.47
Hair dyes are categorized as permanent, semi-permanent, and temporary (Table 4). Permanent dyes are the most widely used due to their durability and wide color range; however, some components have been associated with allergic reactions, toxicity, carcinogenesis, and hair fiber damage. Temporary dyes do not penetrate the cuticle and are removed with washing.48,49
Characteristics of hair dyes.
| Type of dye | Characteristics | Duration |
|---|---|---|
| Temporary | a. High-molecular-weight molecules that remain on the cuticle | Removed after the first washes |
| Semi-permanent | a. Small molecules that diffuse into the cortex without binding stably to proteinsb. No ammonia or hydrogen peroxidec. Gray hair coverage<30% | 6–12 washes |
| Demi-permanent | a. Contains 3 agents: developer, coupler, and oxidant; contains ethanolamine or sodium carbonate instead of ammoniab. Hydrogen peroxide 2%c. Gray hair coverage up to 50% | 20–25 washes |
| Permanent | a. Contains 3 agents: developer, coupler, and oxidantb. Penetrate deeply into the cortex, changing hair structurec. Use ammonia and hydrogen peroxide up to 6%d. 100% gray hair coverage | Color fades with washing; requires retouching of new hair growth |
RT1640 is a compound consisting of cyclosporine A, minoxidil, and RT175, a non-immunosuppressive tacrolimus ligand with regenerative capacity. Cyclosporine A is a calcineurin inhibitor that modulates hair growth by prolonging the anagen phase, activating inactive melanocytes, and blocking catagen. Minoxidil promotes the transition from telogen to anagen, increasing the duration of anagen. Anderson et al. tested this compound in mice and found an 80% increase in melanocyte progenitor cells in the hair bulb, resulting in hair pigmentation even after shaving.50
Saha et al. described the use of a placental extract rich in C18:0 sphingolipids, which activates the microphthalmia-associated transcription factor (MITF) to stimulate quiescent melanocyte stem cells in gray-haired mice, resulting in hair pigmentation and suggesting reactivation of melanocyte stem cells.51
Other drugsNumerous drugs used for other conditions have been identified as causing hair repigmentation. However, not all patients exposed to these drugs develop pigmentation, and hair color usually returns to baseline after drug discontinuation (Table 5).52
Drugs causing hair hyper- or hypopigmentation.
| Depigmentation | Hyperpigmentation/gray hair pigmentation or depigmentation | Hyperpigmentation |
|---|---|---|
| Chloroquine | Imatinib | Cyclosporine |
| Interferon | Valproate | Indinavir |
| Tamoxifen | Cisplatin | Zidovudine |
| Hydroxychloroquine | Etretinate | Verapamil |
| Sunitinib | Acitretin | Para-aminobenzoic acid |
| Pazopanib | ||
| Phenobarbital | ||
| Phenytoin | ||
| Dasatinib |
Hair disorders have a significant impact on quality of life, and PG is no exception. Daulatabad et al. assessed quality of life using the Dermatology Life Quality Index (DLQI) in 57 patients with PG, finding that 65.4% had a significant impact and 19.23% had an extremely significant impact, with high levels of guilt and mood changes.53 Mathias et al. reported that among 100 patients with PG, 49% had a significant impact and 22% an extremely significant impact on quality of life.54 Parihar et al. assessed body image in 295 patients with PG and found poor body image in 54%.55
Conflict of interestThe authors declare that they have no conflict of interest.
Uncited reference43.