We report the case of a patient who developed sarcoid granulomas 11 months after starting treatment with pegylated interferon alfa and ribavirin for chronic hepatitis C. The sites of the lesions were related to 3 different foreign bodies: silica in old scars on the skin, hyaluronic acid that had been injected into facial tissues, and silicone in an axillary lymph node draining the area of a breast implant. Systemic sarcoidosis was diagnosed on the basis of a history of dry cough and fever and blood tests that revealed elevated angiotensin converting enzyme and liver enzymes. Interruption of the antiviral therapy led to normalization of liver function tests and disappearance of the skin lesions and lymphadenopathies. Dermatologists and cosmetic surgeons should be aware of the risk of sarcoid lesions related to cosmetic implants in patients who may require treatment with interferon in the future.
Se describe el caso de una paciente que desarrolló granulomas sarcoideos 11 meses después de haber iniciado interferón α pegilado y ribavirina, como tratamiento de la hepatitis crónica C. Las lesiones se localizaban en relación a 3 cuerpos extraños diferentes: sílice en cicatrices cutáneas antiguas, ácido hialurónico que se había inyectado previamente en la cara, y silicona que se detectó en una adenopatía axilar donde había drenado de un implante mamario previo. La paciente también aquejaba tos seca, fiebre y en la analítica se detectó un incremento de la enzima convertidora de angiotensina y de las enzimas hepáticas. A partir de estos hallazgos se diagnosticó de sarcoidosis sistémica y se suspendió el tratamiento antiviral con posterior normalización de las pruebas hepáticas, desaparición de las lesiones cutáneas y de las adenopatías.
Los dermatólogos y cosmetólogos deben ser conscientes del riesgo de aparición de manifestaciones sarcoideas en las áreas donde se han realizado implantes estéticos, en los sujetos que en un futuro requieran tratamiento con interferón.
Sarcoidosis is more prevalent in patients receiving interferon than in the general population.1,2 Furthermore, the appearance of granulomas in the skin is known to be related to the presence of foreign bodies.3 We describe the case of a patient with chronic hepatitis C who developed sarcoidosis while receiving treatment with pegylated interferon alfa combined with ribavirin. The sarcoid granulomas were located in the skin and were associated with 3 different foreign bodies: silica, hyaluronic acid, and silicone within a lymph node.
Case DescriptionThe patient was a 54-year-old woman who was referred to the dermatology department in September 2009 with erythematous-violaceous nodules on her face.
Important findings in her past history included silicone gel implants to both breasts 19 years previously (these implants had not been replaced) and chronic infection by hepatitis C virus (HCV), genotype 1b, which had been detected 11 years earlier, giving rise to severe liver fibrosis. In September 2008 she started treatment with subcutaneous interferon alfa 2a (180μg weekly) combined with oral ribavirin (1000mg/d), which led to a partial early virologic response. Erythropoietin (40 000IU) was added at week 4 owing to anemia secondary to antiviral treatment.
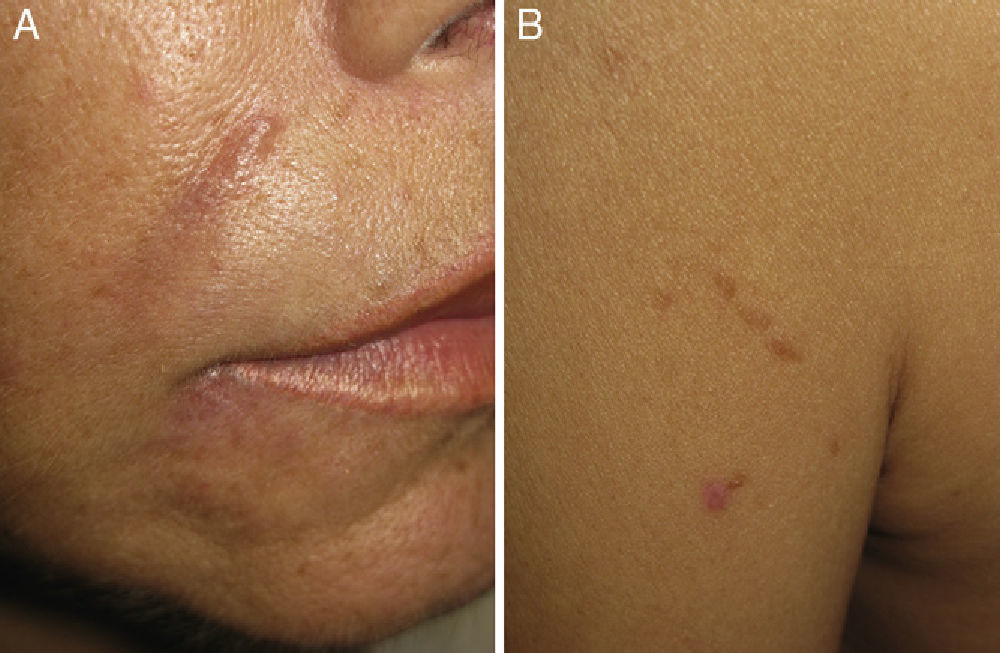
Physical examination revealed erythematous-violaceous nodules in the interciliary sulci and nasolabial folds (Fig. 1, A and B). The patient reported that she had had hyaluronic acid injections at these sites 1 year earlier. She had received treatment with intralesional corticosteroids but had shown no signs of improvement. In addition, she presented nodules covered by skin of normal appearance on both sides of her forehead in areas where she denied having had injections of hyaluronic acid. She also presented brownish papules in a linear distribution on her left shoulder (Fig. 1B), elbows, knees, and in the proximity of old scars, and the axillary lymph nodes were palpable bilaterally. The patient reported having had dry cough and fever (38°C) during the previous month. Laboratory tests revealed the following important results: hemoglobin, 10.1g/dL; white cell count, 2330 μL; and angiotensin-converting enzyme, 80U/L (reference range, 20-60 U/L). The results of the other laboratory tests—including renal and liver function and blood and urinary calcium and phosphate—were within the normal range.
Computed tomography of the chest and abdomen revealed changes in the breast implants, which had acquired a nodular morphology, and bilateral axillary lymphadenopathies, as well as a 1-cm nodule in segment IV of the liver, together with 2 other hepatic lesions measuring <1cm that were suggestive of cysts. Magnetic resonance of the breasts revealed an intracapsular rupture of the implant on the left side.
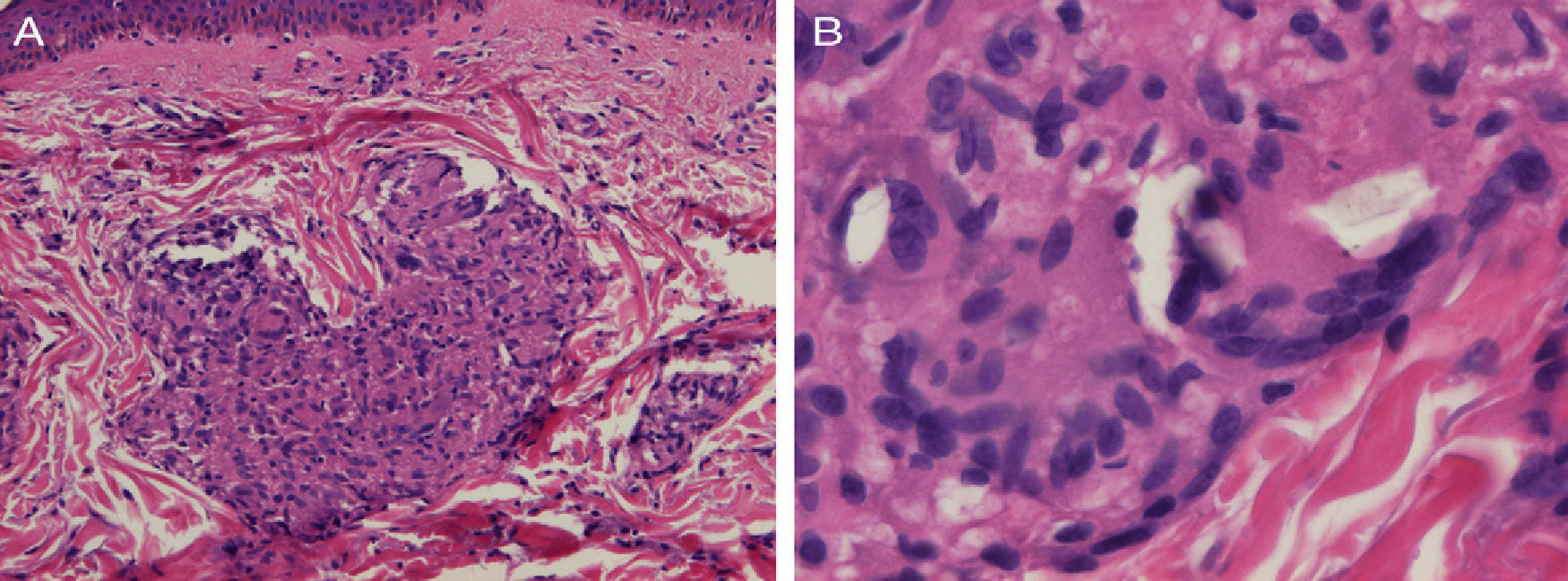
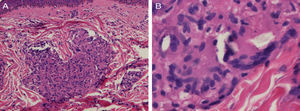
Biopsy of a papule on the left shoulder showed epithelioid granulomas with no central necrosis in the papillary and upper reticular dermis, surrounded by sparse lymphocytes (Fig. 2A). Shiny particles were observed in the cytoplasm of some histiocytes and of some multinucleated giant cells (Fig. 2B). These particles were birefringent with polarized light and were interpreted to be silica.
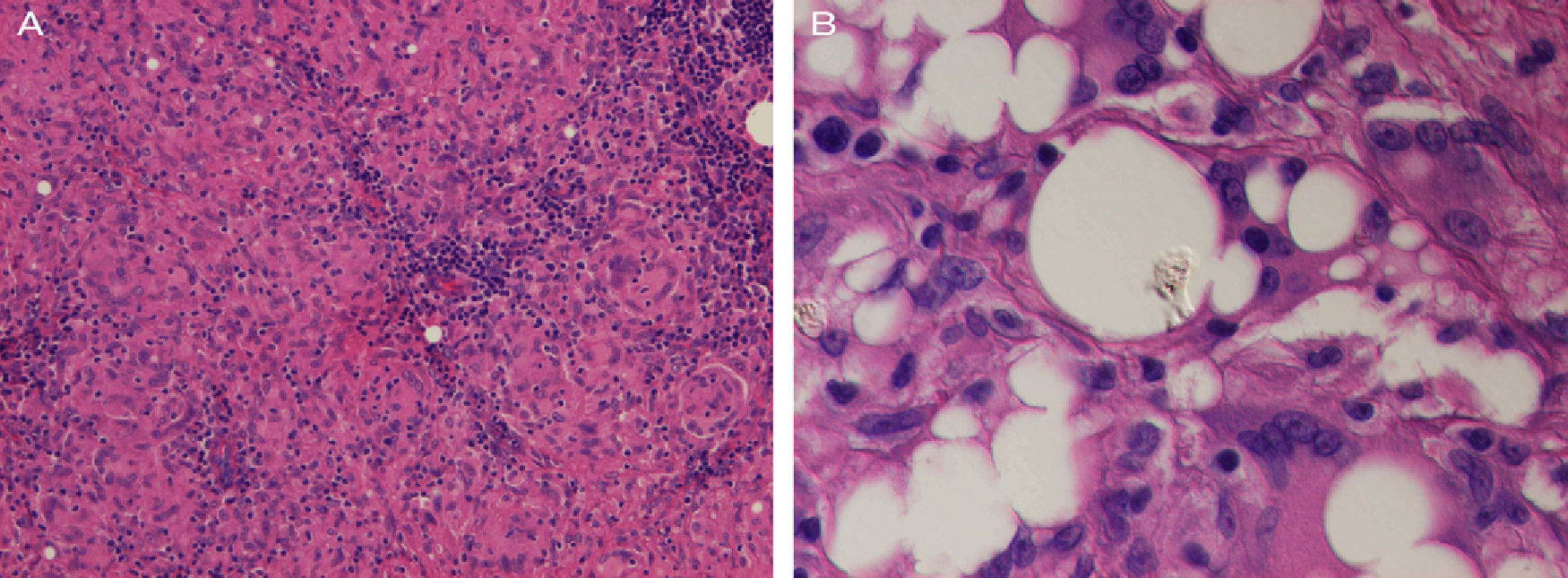
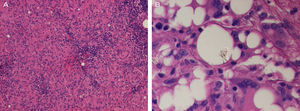
Histopathology of an axillary lymph node revealed numerous nonnecrotizing sarcoid epithelioid granulomas (Fig. 3A). In addition, clear extracellular vacuoles of different sizes were present in the center of the node and there were abundant multinucleated giant cells with cytoplasmic vacuoles. Nonbirefringent refractile material was observed inside some vacuoles. Given that the patient had silicone breast implants, this finding was compatible with silicone from the implants (Fig. 3B), thus indicating silicone lymphadenopathy.
A, Histopathology of the left axillary lymph node showing widespread sarcoid granulomas (hematoxylin and eosin, original magnification, ×200). B, The center of the node contains clear vacuoles in the extracellular space and in the cytoplasm of multinucleated giant cells. Note the refractile material within these vacuoles (hematoxylin and eosin, original magnification, ×400).
A diagnosis of sarcoidosis was made. As no visceral involvement was observed, the same antiviral treatment was maintained. However, in November 2009, hepatic cholestasis was detected (alkaline phosphatase 245U/L [reference range, 35-110 U/L] and gamma-glutamyltransferase 469U/L [reference value,<43 U/L]) and treatment with pegylated interferon alfa 2a and ribavirin was stopped. The results of the laboratory tests subsequently returned to normal values, the size of the cutaneous lesions gradually decreased, and the cough and fever resolved. In addition, the virologic response was maintained 1 year after completing 60 weeks of antiviral treatment.
DiscussionSarcoidosis is a multisystem granulomatous disease of unknown etiology that is sometimes associated with interferon administered to treat other diseases.4–7
Sarcoidosis is detected more frequently in HCV-infected patients than in the general population. In their 2010 study, Faurie et al.2 found a prevalence of 0.12% in a series of HCV-infected patients; in the general population, prevalence has been estimated to be between 1 and 40 cases per 100 000.1 In 2005, Ramos-Casals et al.1 reviewed 68 cases of sarcoidosis associated with chronic HCV infection and compared their findings with a control group of non–HCV-infected patients. Those authors found that skin involvement in sarcoidosis was more frequent in HCV-infected patients (56%) than in non–HCV-infected patients (22%). In 4 cases, the granulomas were adjacent to old scars and tattoos. Faurie et al. considered that sarcoidosis had been triggered by antiviral treatment in most cases (79.4%). The potential role of interferon could arise from the induction of a type 1 helper T-cell response, which is thought to affect the formation of granulomas in patients with sarcoidosis.2 Both pegylated and nonpegylated interferon alfa, whether in monotherapy or in combination with ribavirin, have been cited as possible triggers of sarcoidosis. Prognosis is generally good in these cases, and sarcoidosis can resolve spontaneously in 85% of cases if antiviral treatment is stopped.1 In the case we report, antiviral treatment was maintained until the onset of systemic manifestations. At that time, antiviral therapy was discontinued, even though the 72-week regimen had not been completed.
In the present case, we think that sarcoidosis was probably induced by interferon and ribavirin for 2 reasons: first, the lesions appeared 11 months after starting treatment, and second, the skin and liver lesions, the liver function tests, angiotensin-converting enzyme levels, and changes in the breast implants returned to normal once interferon was discontinued.
We believe that the case we report is unusual, as the sites of the sarcoid granulomas were associated with the presence of 3 foreign bodies (silica, hyaluronic acid, and silicone). The simultaneous onset of cutaneous sarcoid granulomas associated with 2 foreign bodies (silica and silicone) has been described elsewhere.8 Of note, Marcoval et al.3 demonstrated the presence of foreign bodies in cutaneous granulomatous lesions in 22% of 65 patients with systemic sarcoidosis and skin involvement and suggested that these foreign bodies could induce the formation of granulomas in patients with sarcoidosis.
There have been reports of systemic sarcoidosis associated with tattoos9 and fillers (permanent and reabsorbable).10–12 Two studies11,12 have reported sarcoid granulomas associated with injection of hyaluronic acid in 2 women: one of those patients had hepatitis C treated with interferon.11
We report the first case of sarcoid granulomas developing in a silicone-induced lymphadenopathy. This complication of breast implants is caused by migration of silicone to the lymph nodes.13 Sarcoidosis has been reported in patients with breast implants.14,15 The condition improved spontaneously in 1 case,14 and in the other, the patient's condition improved dramatically after removal of the implants.15
We believe that sarcoidosis associated with HCV infection is of special interest for the dermatologist, since it frequently affects the skin (56% of cases). In addition, cosmetic procedures such as tattoos and the use of fillers may induce the appearance of sarcoid granulomas in patients who develop sarcoidosis. It has been suggested that the informed consent signed before cosmetic microimplant procedures should include the risk of severe interaction between the injected material and interferon or other immunostimulants, were the patient to need such treatments in the future.10 This warning should be given even before injection of reabsorbable material.
Any changes in the areas where cosmetic materials have been injected should alert the dermatologist and cosmetic surgeon to a possible diagnosis of sarcoidosis, especially in patients with HCV infection. Furthermore, before receiving cosmetic injections, patients should be warned of the risk of developing or reactivating sarcoidosis in the future.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Novoa R, Barnadas MA, Torras X, Curell R, Alomar A. Reacción granulomatosa a cuerpo extraño a sílice, silicona y ácido hialurónico, en paciente con sarcoidosis inducida por interferon. Actas Dermosifiliogr. 2013;104:920–923.