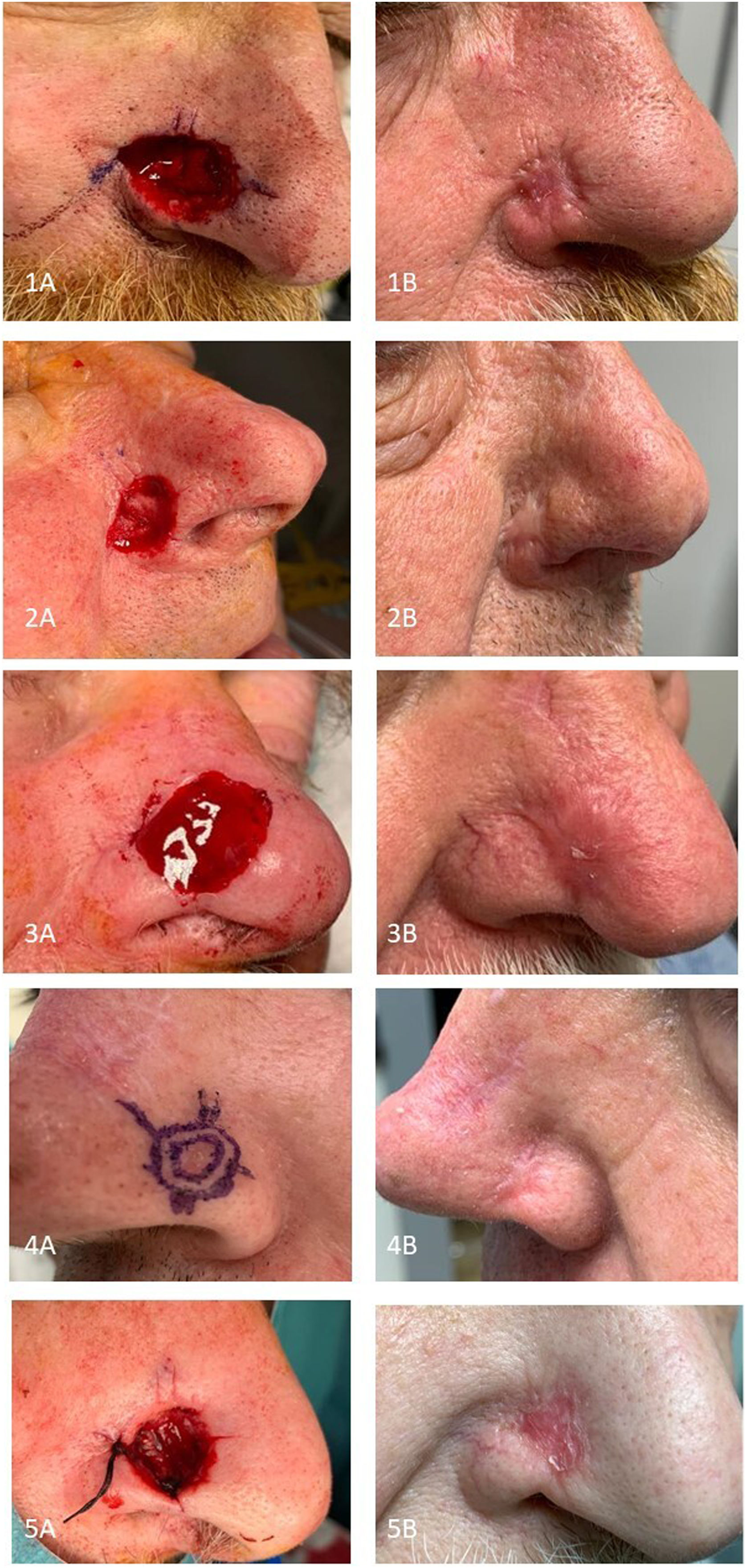
The reconstruction of surgical defects of the nasal ala is dermatologically challenging due to its particular morphology, rigidity, sebaceous nature, and centrofacial location. We present a series of 5 patients with postoperative defects in the nasal ala, reconstructed using a dermal fat graft (DFG) (Table 1) composed of dermis and its underlying fat. Although its use in dermatology is more recent,1 it is a technique widely used in other specialties to correct defects with volume loss in the facial area.2,3
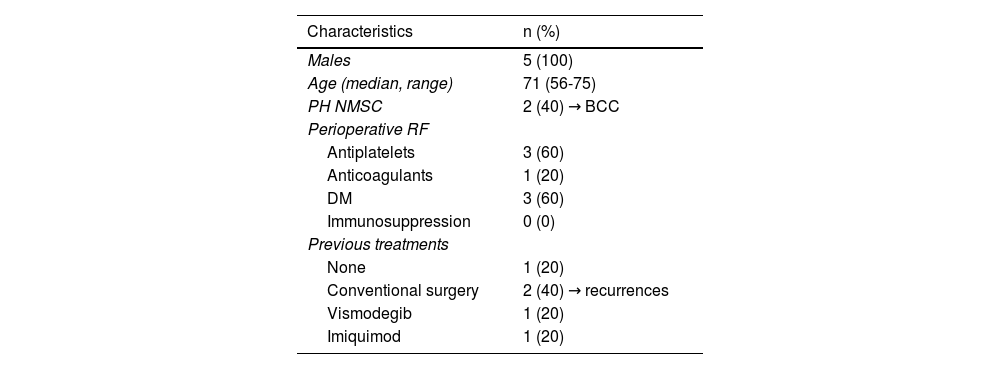
Description of the Characteristics of the 5 Treated Patients.
| Characteristics | n (%) |
|---|---|
| Males | 5 (100) |
| Age (median, range) | 71 (56-75) |
| PH NMSC | 2 (40) → BCC |
| Perioperative RF | |
| Antiplatelets | 3 (60) |
| Anticoagulants | 1 (20) |
| DM | 3 (60) |
| Immunosuppression | 0 (0) |
| Previous treatments | |
| None | 1 (20) |
| Conventional surgery | 2 (40) → recurrences |
| Vismodegib | 1 (20) |
| Imiquimod | 1 (20) |
n: sample size; PH NMSC: personal history of non-melanoma skin cancer; BCC: basal cell carcinoma; FR: risk factors; DM: diabetes mellitus.
Patients underwent Mohs surgery for basal cell carcinoma on the nasal ala (Table 1), and reconstruction using DFG. The donor area was the forearm anterior region, as it was easily accessible during surgery. Other alternatives include the supraclavicular fossa, lower abdominal region, groin, or mid-sacral region, with preference for areas with abundant adipose tissue, skin laxity, fine hair, and low visibility.1–3 For reconstruction purposes, we designed a square of similar size to the defect and performed an incision with a cold scalpel, deepening to the adipose panniculus, leaving one side of the square uncut, which would act as a pedicle. Afterwards, the graft was de-epithelialized, and a plane in the subcutaneous tissue was created using dissection scissors, cutting the pedicle of dermis and fat to release the graft. We adapted its shape to the alar defect, sutured it, and applied a silicone contact sheet and a knotted moist dressing. To promote stretching of the nasal ala skin and prevent retraction, we placed a nasal packing and replaced it every 48hours at their health center. We conducted the first postoperative assessment 7 days after surgery, at which point the knotted dressing and nasal packing were removed. The serohematic scab was removed in cases where it was present, and moist healing with antibiotic ointment was performed. Patients continued with ambulatory moist dressings every 48hours, with weekly follow-ups at our service to remove any serohematic scab that might appear. One month after surgery, all 5 patients showed complete healing of the defect, with satisfactory functional and aesthetic results, and a median re-epithelialization time of 25 days (range, 21-37) (Fig. 1).
The most common reconstructive options for nasal ala defects include local flaps, full-thickness skin grafts (FTSG), or healing by secondary intention. However, these techniques present various drawbacks. Local flaps often involve adjacent anatomical units, leading to unsightly scars and nasal asymmetry. Although FTSG or secondary intention address the previous problems they do not guarantee optimal color or volume, with the latter showing prolonged healing times that are bothersome for the patient.4,5 In contrast, DFG offers certain advantages: the dermal component provides vascularization and proangiogenic factors, while the adipose tissue supplies stem cells and anti-inflammatory cytokines that, along with the former, promote healing and provide volume and support to the graft. By re-epithelializing from the skin adjacent to the graft, an optimal tone and texture is ensured with a shorter healing time than secondary intention. The support and volume qualities of DFG could be of interest not only in the reconstruction of nasal area defects but also in other anatomical units, for both primary reconstructions of deep defects and the correction of volume defects from previous surgical procedures, being a simple technique with easily available donor tissue.1–3 Its angiogenic capacity makes it potentially viable in the presence of scar tissue, being particularly useful in recurrence surgical procedures or areas treated with radiotherapy.2,5 It is also useful in surgical procedures without margin control, as it does not alter the nasal morphology if affected margins are present. Although it does not have specific absolute contraindications, DFG is not recommended in the presence of severe infection or inflammation of the surgical bed that could compromise its viability. In maxillofacial surgery, the most reported complications are unpredictable graft resorption (15% up to 45% of cases) and the development of epidermal inclusion cysts, which can be avoided by using donor areas without hair or with fine hair.2 Seroma, hematoma, or infection have also been reported at the donor or recipient sites.3 Only 1 out of our 5 patients developed a small seroma at the donor site, which resolved spontaneously, with no other adverse events reported. Alar retraction has also been reported, which can be mitigated by early removal of the scab that forms over the graft.1 We propose the use of nasal packing, although studies with a larger numer of patients would be needed to corroborate its effectiveness.
In conclusion, DFG can be considered an effective alternative for nasal ala reconstruction, with satisfactory aesthetic and functional results. Given its low technical difficulty, high viability, and availability of donor tissue, it may be particularly useful in recurrence surgical procedures, in areas previously treated with radiotherapy, or in surgical procedures without margin control.