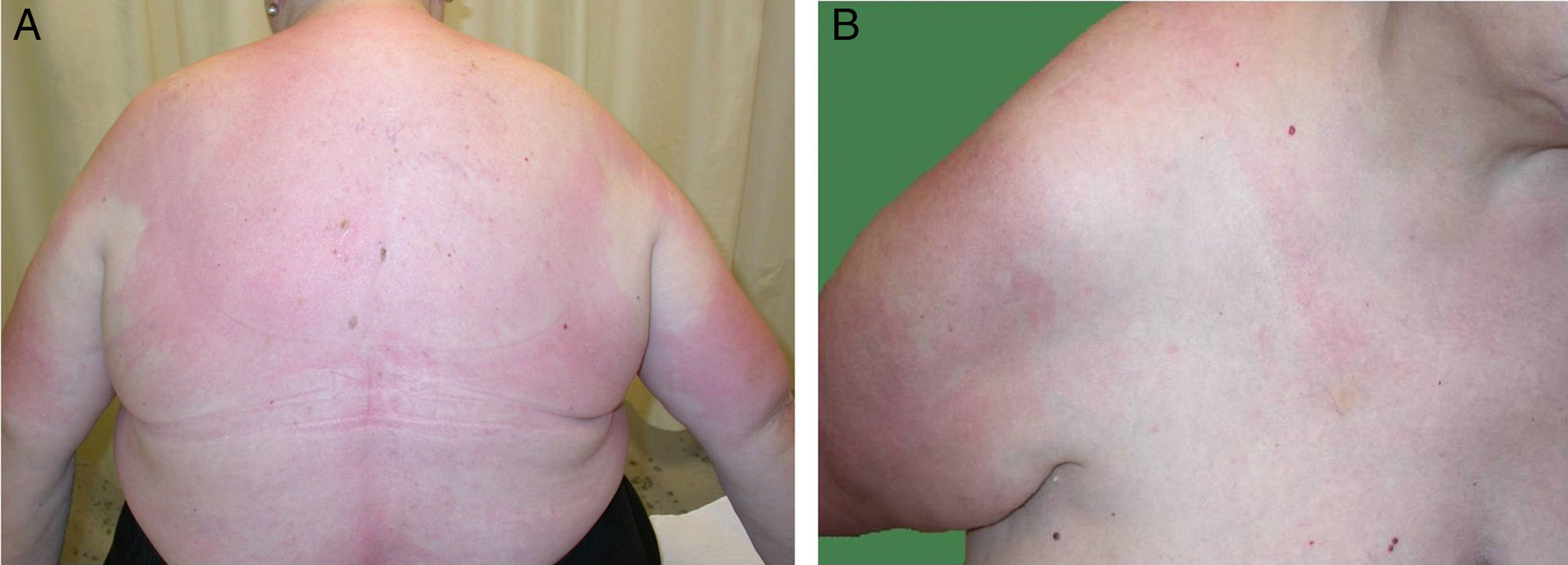
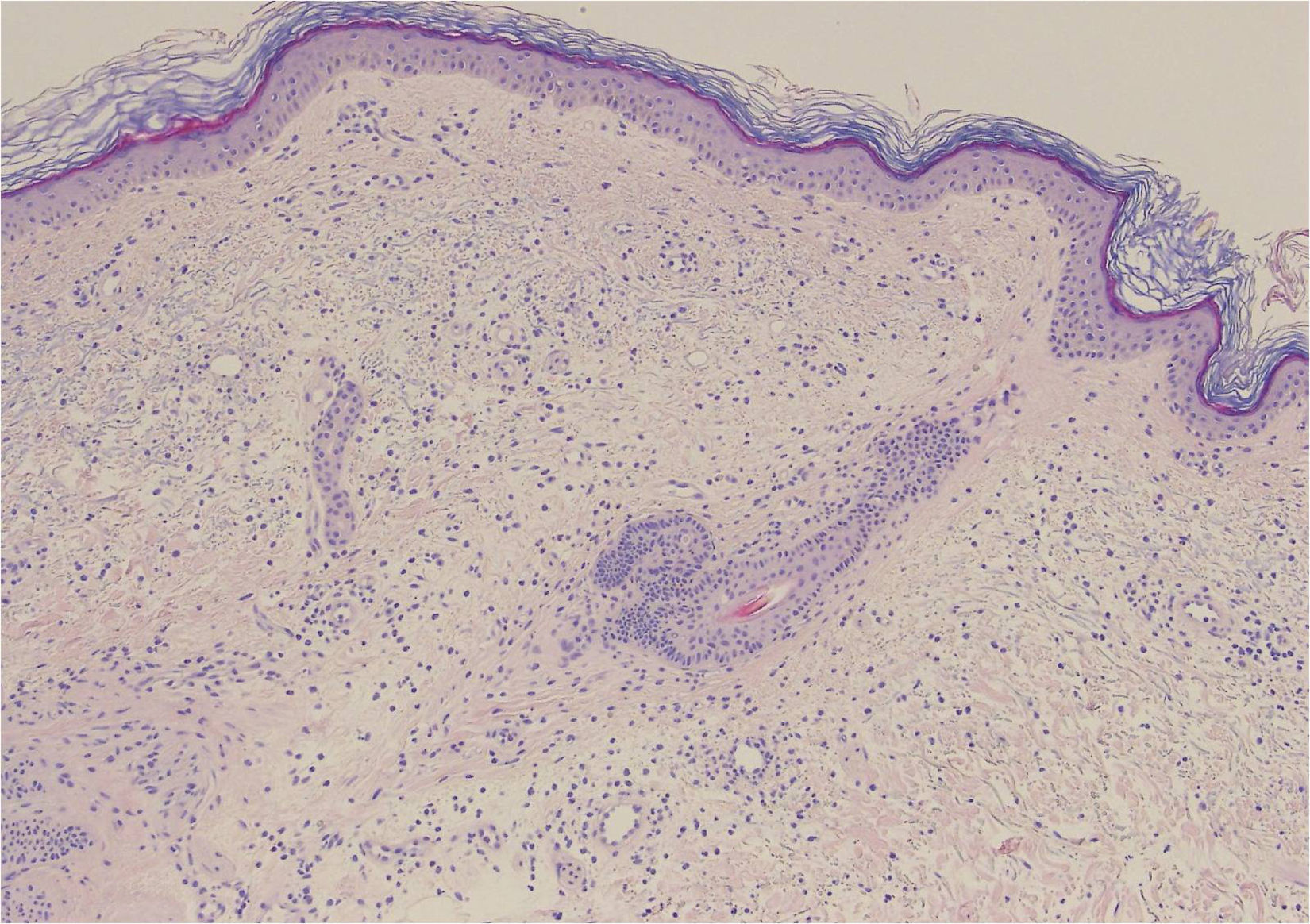
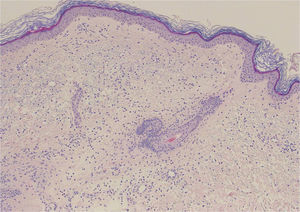
A 68-year-old woman with a history of neurosensorial deafness and anemia of chronic disease consulted for more than 20 years of recurrent episodes of fever, joint and muscle pain, and skin lesions on the arms and trunk. The episodes occurred every 2 to 6 months and lasted around 2 weeks. She was asymptomatic between episodes. Large edematous erythematous plaques arose on her trunk and the root of her upper limbs (Fig. 1), associated with fever of 38°C. Blood tests were normal. Skin biopsy showed edema and an inflammatory infiltrate of lymphocytes and neutrophils in the superficial dermis, without fibrinoid necrosis (Fig. 2).
Edema associated with a moderate inflammatory infiltrate of lymphocytes and neutrophils in the superficial dermis, with occasional images of leukocytoclasia. No extravasation of red blood cells is observed, nor the presence of hemosiderophages or lesions of fibrinoid necrosis in the vessel walls. Hematoxylin and eosin, original magnification×20.
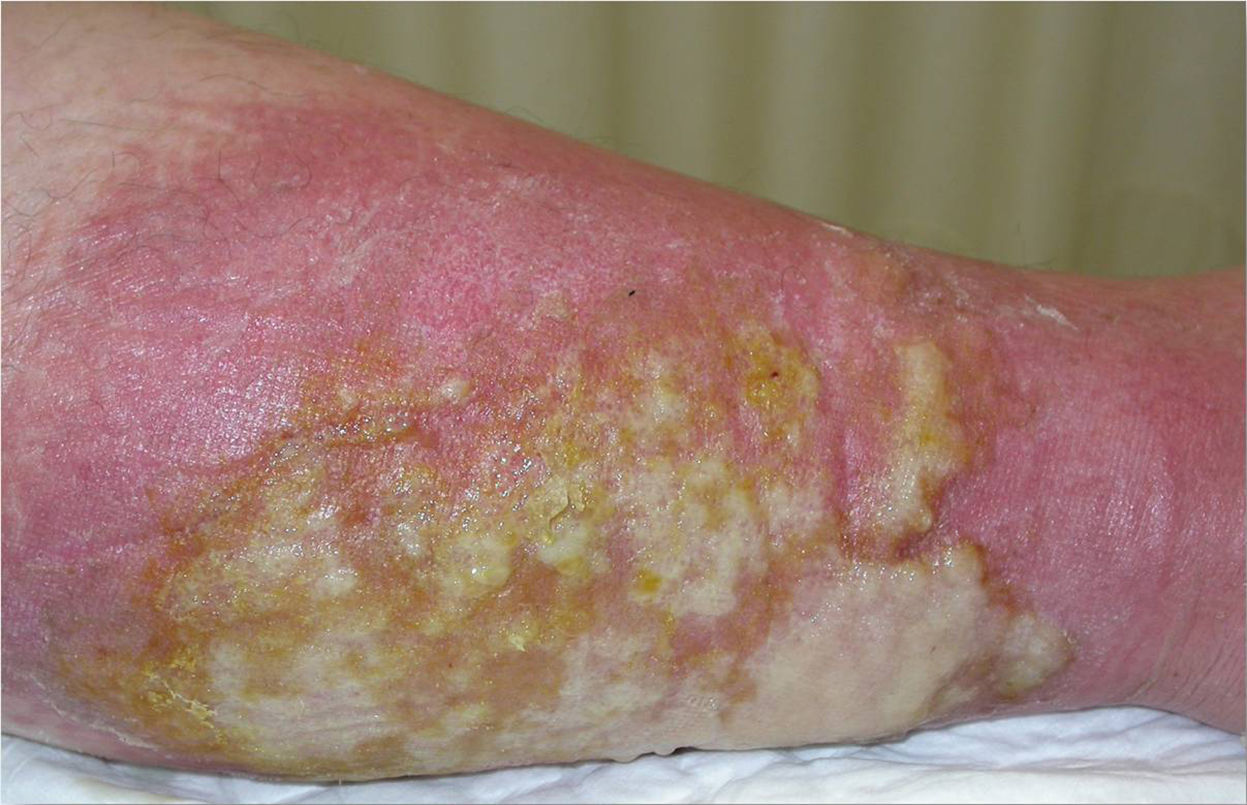
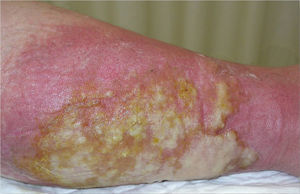
She subsequently developed fever associated with erythema and increased temperature in 1 of her legs, with blisters and an abundant exudate (Fig. 3). A gram-negative microorganism was isolated on culture, and this was interpreted as infectious cellulitis. Four months later she presented similar manifestations in her other leg.
Genetic analysis was performed of genes MEFV, TNFRSF1A, MVK, NLRP3, NOD2, and PSTPIP1. The patient was heterozygous for the c.1772T>C variant of gene MEFV.
As she also satisfied the Tel-Hashomer diagnostic criteria for familial Mediterranean fever (FMF) (Table 1), she was diagnosed with this entity. Treatment was started with colchicine, which led to a marked improvement in the clinical manifestations.
Tel-Hashomer Diagnostic Criteriaa
| Major Criteria | Minor Criteria | Data That Support the Diagnosis |
|---|---|---|
| Typical episodes: recurrent, <72hours, febrile 1. Diffuse peritonitis 2. Unilateral pleuritis or pericarditis 3. Monoarthritis of the lower limbs 4. Fever as the only manifestation | Atypical episodesb: no fever, longer duration, other manifestations different from 1-3 of the Major Criteria 4. Pain in the lower limbs on exertion 5. Favorable response to colchicine | 1. Family history of FMF 2. Typical ethnic origin 3. Onset<20 years Characteristics of the episodes: 4. Marked alteration of the general state, requiring rest 5. Spontaneous remission 6. Symptom-free between episodes 7. Elevation of at least 1 AFR: fibrinogen, white cell count, ESR 8. Episodes of proteinuria or hematuria 9. Acute abdomen with negative exploratory laparotomy 10. Consanguinity |
FMF is the most common autoinflammatory disease of adults.1 It has a monogenic autosomal recessive inheritance, caused by a mutation in gene MEFV (16p13.3), although 20% of patients are heterozygous.2 This could be because it is actually an autosomal dominant disease with variable penetrance, it was not possible to detect the second mutation due to technical limitations, or that it could be a polygenic disease. Some authors have stated that these patients have a later onset of the disease, with a shorter duration of outbreaks, milder symptoms, and longer symptom-free intervals.3
Gene MEFV codes the protein pyrin, also known as marenostrin, which plays a role in regulation of the innate immune response. Alteration of this protein removes control from the pathway, increasing the levels of interleukin 1β, responsible for the inflammatory response in this disease.4
Soriano and Manna5 described 4 clinical phenotypes of the disease:
- –
Type 1: Patients with recurrent episodes of short duration (12-72h) with fever, acute abdominal pain, joint involvement, acute chest pain due to pleuritis/pericarditis, and various skin manifestations. Patients are usually asymptomatic between outbreaks, although biochemical evidence of the disease can be detected.
- –
Type 2: Patients who develop secondary amyloidosis (AA type) with proteinuria or kidney failure before they develop other signs of FMF, or as the only manifestation in relatives of patients with FMF.
- –
Type 3: Carriers of a mutation of gene MEFV with no clinical manifestations of the disease and no amyloidosis. This occurs in endemic populations (such as Iraqi Jews and Ashkenazi Jews), with a prevalence of 1 in 25 to 1 in 300 persons. Although the majority never present clinical manifestations, some do develop amyloidosis with time (phenotype2).
- –
FMF-like: Heterozygous patients with mild clinical manifestations of the disease. Our patient falls into this phenotype.
Cutaneous manifestations of the disease occur in 10% to 40% of affected patients.6 Only erysipelas- or cellulitis-like lesions, seen in 5% to 30% of patients with FMF, are considered specific, and they arise on the anterior surface of the legs and dorsum of the feet. Histology reveals a perivascular dermal infiltrate made up mainly of mononuclear cells and neutrophils, but with no vasculitis.7 The episodes that affected our patient's legs probably corresponded to this clinical presentation. Panniculitis can present as erythema nodosum or neutrophilic panniculitis. Urticarial manifestations are more typical of other autoinflammatory syndromes, such as TRAPS (tumor necrosis factor receptor associated periodic fever syndrome) and CAPS (cryopyrin-associated autoinflammatory syndromes).8
The differential diagnosis should include other autoinflammatory diseases.8 In our case, we considered Muckle-Wells syndrome because of the urticarial lesions and neurosensorial deafness, although the genetic analysis finally confirmed the diagnosis of FMF. Some authors believe that patients with FMF may have a greater prevalence of auditory loss than the unaffected population, but there has been no evidence of this in any controlled study.9
The diagnostic criteria of FMF were established in 1997 (Table1).10 The diagnosis is clinical; genetic analysis is only used for support. In our opinion, genetic analysis may be fundamental to reaching the diagnosis in some cases. However, detection of a mutation cannot alone confirm the diagnosis, particularly in endemic populations in which prevalence of the mutation is very high.
The main objectives of treatment are the prevention of outbreaks and, in the long-term, of secondary amyloidosis. The drug of choice is colchicine, 1-3mg/d.4
In conclusion, in patients with recurrent episodes of fever with cutaneous manifestations, we should consider the autoinflammatory syndromes, which include FMF. The diagnosis is basically clinical, although genetic analysis can be very useful. This disease is classically considered to have autosomal recessive inheritance, although a notable percentage of heterozygous patients have symptoms, meaning that aspects of the genetics of this disease still remain to be clarified.
Please cite this article as: Pascual LL, García ML, Chávarri CL, Bayona JIY. Fiebre mediterránea familiar. Dificultades diagnósticas en un caso atípico. Actas Dermosifiliogr. 2017;108:161–164.