Extracorporeal photopheresis (ECP) is an immunomodulatory therapy used to treat graft-vs-host disease (GVHD) in adults and children. Few studies have examined its use in children.
ObjectiveTo describe demographic characteristics, clinical response, adverse effects, and outcomes in a series of pediatric patients with acute or chronic GVHD treated with ECP.
Material and methodsWe included all pediatric patients with acute or chronic GVHD treated with ECP by the dermatology department of Hospital Italiano de Buenos Aires between January 2012 and December 2018. We used the UVAR-XTS™ system (2 patients) and the CELLEX system (7 patients). Patients with acute GVHD received 2 sessions a week and were reassessed at 1 month, while those with chronic GVHD received 2 sessions every 2 weeks and were reassessed at 3 months. Treatment duration in both scenarios varied according to response.
ResultsWe evaluated 9 pediatric patients with corticosteroid-refractory, -dependent, and/or -resistant GVHD treated with ECP. Seven responded to treatment and 2 did not. Response was complete in 1 of the 9 patients with skin involvement and partial in 7. Complete response rates for the other sites of involvement were 60% (3/5) for the liver, 50% (1/2) for the gastrointestinal system, and 80% (4/5) for mucous membranes. Two patients died during the study period.
ConclusionECP is a good treatment option for pediatric patients with acute or chronic GVHD.
La fotoaféresis extracorpórea (FEC) es una terapia inmunomoduladora indicada para la enfermedad injerto contra huésped (EICH) en adultos y niños, no obstante existen pocos estudios en esta última población.
ObjetivoDescribir las características demográficas, respuesta clínica, efectos adversos y evolución de pacientes pediátricos con EICH aguda (EICH-a) y EICH crónica (EICH-c) tratados con FEC.
Materiales y métodosSe incluyeron todos los pacientes con EICH-a y EICH-c sometidos a tratamiento con FEC entre enero de 2012 y diciembre de 2018 en el Servicio de Dermatología del Hospital Italiano de Buenos Aires. Se utilizó el sistema UVAR-XTS™ en dos pacientes y el CELLEX™ en el resto, con dos sesiones por semana y reevaluación al mes en EICH-a, dos sesiones cada dos semanas con reevaluación a los tres meses en EICH-c, y en ambos finalización según respuesta.
ResultadosEvaluamos 9 pacientes pediátricos con EICH refractaria, dependiente y/o resistente a corticoides sistémicos tratados con FEC. Siete pacientes fueron respondedores y 2 no respondedores. La piel presentó respuesta completa (RC) en 1/9 y respuesta parcial en 7/9 pacientes, el hígado, el sistema gastrointestinal y las mucosas presentaron RC en 3/5, 1/2 y 4/5 pacientes, respectivamente. Dos pacientes fallecieron durante el periodo estudiado.
ConclusiónLa FEC es una buena opción terapéutica para los pacientes pediátricos con EICH aguda y crónica.
Graft-vs-host disease (GVHD) is a severe and frequent complication of allogenic bone-marrow transplant (ABMT) and is the main cause of morbidity and mortality in these patients. The organs principally involved are the skin, liver, and gastrointestinal system.1
First-line treatments are corticosteroids and calcineurin inhibitors. Extracorporeal photopheresis (ECP) is an immunomodulatory therapy indicated for acute and chronic GVHD in adults and children, and is considered a second-line treatment in GVHD resistant to, dependent on, or intolerant of systemic corticosteroids or immunosuppressants. ECP is well tolerated in all age groups, although the pediatric population is more vulnerable to small changes in volume than the adult population. Thus, in children, extracorporeal volume, access to a vein, weight (under 30 kg), and the use of anticoagulants in the procedure are factors that pose a major challenge to treatment of these patients.2–6
Few studies have been published exclusively on pediatric patients. This study describes our experience with ECP in 9 pediatric patients with GVHD and the objectives of the study were to identify the clinical characteristics of those patients with acute or chronic GVHD treated with ECP, and to determine the clinical response, adverse effects, and course.
Material and MethodsWe designed an observational, descriptive study that included all patients under 18 years of age with a diagnosis of acute or chronic GVHD treated with ECP between January 2012 and December 2018 at the dermatology department of Hospital Italiano, Buenos Aires, Argentina.
Inclusion Criteria-
Age: 18 years of age or younger
-
Acute GVHD grade II-IV (according to Rowlings et al 1997)7
-
Chronic GVHD from moderate to hepatic disease, according to the classification of Jagasia et al 20141
-
Patients who had undergone treatment for at least 1 month in acute GVHD and 3 months in chronic GVHD
-
Confirmed diagnosis of GVHD refractory to, dependent on, or intolerant of corticosteroids (Table 1)
Table 1.Definition of Response to Corticosteroids.4,12,20
Refractory a-GVHD: lack of response to prednisone after a week of treatment at a dosage of 2-5 mg/kg/d Refractory c-GVHD: lack of clinical response at a dosage of 1 mg/kg/d of prednisone for 2 weeks or stable disease with at least 0.5 mg/kg/d for 4 to 8 weeks Dependence: difficulty reducing prednisone to below 0.5 mg/kg/d Intolerance: adverse effects to corticosteroids such as hyperglycemia, osteopenia, high blood pressure, and others Abbreviations: GVHD indicates graft-vs-host disease; a-GVHD, acute graft-vs-host disease; c-GVHD, chronic graft-vs-host disease.
Response evaluation was performed in line with the criteria of Greinix et al 19988 (Table 2).
Evaluation of Study Objectives According to the Criteria of Greinix et al.
| CR: complete resolution of organic manifestations. |
| PR: greater than 50% response in the organs involved. |
| SD: less than 50% response in the organs involved. |
| PD: no response or inability to reduce immunosuppressants, or appearance of new lesions in the organs involved. |
| Responders: patients with CR or PR. |
| Nonresponders: patients with SD or PD. |
Abbreviations: CR indicates complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Source: Greinix et al.8
In acute GVHD, 2 sessions were provided per week for 4 weeks, with re-evaluation after 1 month and end of treatment at 3 months or at the time of maximum response. In chronic GVHD, 2 sessions were provided every 2 weeks, with re-evaluation after 3 months and end of treatment depending on response. The Therakos UVAR-XTS™ system was used in 2 patients and, due to the change in technology during the study period, the Therakos CELLEX™ device was used in the other patients.
Statistical AnalysisVersion 14.0 of the STATA statistical software package was used to perform the statistical analysis. Continuous variables were reported as mean and interquartile range, according to the observed distribution. Categorical variables were reported as absolute frequency and proportion.
The study was approved by the ethics committee of Hospital Italiano, Buenos Aires, Argentina (Protocol no.: 5.309), and the adult responsible for each pediatric patient signed the corresponding informed consent.
ResultsNine pediatric patients were enrolled between January 2012 and December 2018, of which 5 were girls/adolescents, with a mean age and weight at start of ECP of 15 years (IQR, 12–16 y) and 43 kg (IQR, 39–53.5 kg), respectively. With regard to vascular access, 5 patients required a central venous catheter (CVC), and a peripheral vein was used in 4.
The most common underlying disease for which the transplant was required was acute lymphoid leukemia, followed by bone marrow aplasia, and the most common type of transplant was an unrelated donor transplant (6/9). Seven patients presented chronic GVHD, 1 of whom was corticosteroid-dependent, and 2 patients presented acute GVHD, 1 of whom was dependent on and the rest resistant to corticosteroids. Of the 2 patients with acute GVHD, both presented cutaneous involvement, 1 presented hepatic involvement, and 1 gastrointestinal (GI) involvement. Of the patients with chronic GVHD, all 7 presented cutaneous involvement, 5 presented fascia and muscule (FM) involvement, 5 presented mucosa involvement (oral and ocular), 4 presented hepatic involvement, and 1 presented GI involvement (Table 3).
Clinical Characteristics of Patients Treated with ECP.
| No. | Age, y | Sex | Weight, kg | Initial Diagnosis | Type of Transplant | a-GVHD | c-GVHD | Cutaneous Involvement | Visceral Compromise | Response to Corticosteroids |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | F | 49 | AML | UD | — | S | Sc 2 | H, Oc, FM | CD |
| 2 | 16 | M | 43 | ALL | UD | — | M | Sc 2 | H, Oc, FM | NA |
| 3 | 10 | F | 37 | BMA | UD | — | S | Sc 2 | FM | NA |
| 4 | 4 | M | 32 | ALL | RFM | G II | — | G 3 | — | CD |
| 5 | 16 | M | 43 | BMA | UD | — | M | Sc 2 | H, Oral | NA |
| 6 | 14 | F | 62 | ALL | UD | — | M | Sc 2 | Oral | NA |
| 7 | 15 | F | 41 | ALL | RFM | — | S | Sc 2 | H, GI, Oral; FM | NA |
| 8 | 16 | M | 58 | SCF | UD | — | M | Sc 2 | FM | NA |
| 9 | 15 | F | 46 | BMA | RFM | G III | — | G 2 | GI, H | CR |
Abbreviations: ALL indicates acute lymphoid leukemia; AML, acute myeloid leukemia; BMA, bone marrow aplasia; CD, corticosteroid dependent; CR, corticosteroid resistant; FM, fascia/muscular; G, grade; GI, gastrointestinal; H, hepatic; M, moderate; NA, not applicable; Oc, ocular; RFM, related family member; S, severe; Sc, score; SCF, severe combined failure; UD, unrelated donor.
In terms of response to ECP, 7 patients were responders (R) and 2 were nonresponders (NR); both the nonresponders had acute GVHD. Cutaneous response was partial (PR) in 7 patients, stable (SS) in 1, and complete (CR) in 1. Of the 5 patients with mucosal involvement (all with chronic GVHD), 4 presented a CR and 1 a PR. Of the 5 patients with FM involvement, 3 presented a CR and 2 a PR. Of the 5 patients with hepatic involvement (1 with acute GVHD and 4 with chronic GVHD), 3 presented a CR 1 a PR, and 1 progressive disease (PD). Of the 2 patients with GI involvement (1 with acute GVHD and 1 with chronic GVHD), 1 presented a CR and 1 PD. Median duration of ECP was 7 months (IQR, 3–17.5 mo), 24 sessions (IQR, 19–59 sessions) and the median number of procedures required to obtain a response was 12 sessions (IQR, 8–18 sessions), 7 months (IQR, 3–17.5 mo) (Table 4).
Results of extracorporeal photopheresis in pediatric patients.
| No. | Treatment Duration, mo | Median procedures, sessions | Associated Therapy | a-GVHD | c-GVHD | Response to ECP | Response by Organ | Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutaneous | FM | Hepatic | GI | Oral | Ocular | ||||||||
| 1 | 22 | 90 | FK 506, C, Et | — | S | R | PR | PR | PR | — | — | PR | D |
| 2 | 3 | 22 | FK 506, C | — | M | R | PR | CR | CR | — | — | CR | L |
| 3 | 8 | 36 | FK 506, Et, MPM | — | S | R | PR | PR | — | — | — | — | L-ECP |
| 4 | 2 | 11 | r, s, MPM, C | G II | — | NR | SD | — | — | — | — | — | L-ECP |
| 5 | 5 | 16 | FK 506 | — | M | R | PR | — | CR | — | CR | — | L-ECP |
| 6 | 7 | 23 | FK 506, I | — | M | R | PR | — | — | — | CR | — | L |
| 7 | 13 | 48 | FK 506, I | — | S | R | PR | CR | CR | CR | CR | — | L |
| 8 | 41 | 70 | FK 506 | — | M | R | CR | CR | — | — | — | — | L |
| 9 | 5 | 10 | C, MPM | G III | — | NR | PR | — | PD | PD | — | — | D |
Abbreviations: a-GVHD indicates acute graft-vs-host disease; C, corticosteroids; c-GVHD, chronic graft-vs-host disease; CR, complete response; D, deceased; Et, etanercept; FK 506, tacrolimus; FM, fascia/muscular; G, grade; GI, gastrointestinal; I, infliximab; L, living; L-ECP, living in photopheresis; M, moderate; MPM, mycophenolate mofetil; NR, nonresponder; PD, progressive disease; PR, partial response; R, responder; r, rituximab; S, severe; s, sirolimus; SD, stable disease.
All patients received immunosuppressive treatment during the study (tacrolimus, mycophenolate mofetil, sirolimus, etc.), and 4 patients were also undergoing treatment with systemic corticosteroids. Of the 4 patients who received corticosteroids, 2 were able to reduce the dosage, 33% and 90% of the initial dosage (both patients were corticosteroid-dependent) 2 and 4 months after start of ECP, respectively. One patient was able to suspend immunosuppressant treatment after 12 months of ECP and another suspended immunosuppressant treatment 11 months after starting it. Most of the adverse effects that presented during the study were linked to CVC infections (n, 3), and only 2 patients presented mild low blood pressure, which was corrected with hydration and postural maneuvers.
Of the 9 patients, 2 died during the study period, 1 due to an opportunist infection not associated with the CVC and 1 due to the progression of the underlying disease.
DiscussionABMT is a treatment that has become significantly more frequent in recent years, as it provides an increase in life expectancy for patients with lymphoproliferative diseases. This has increased the incidence of GVHD, making it a considerable diagnostic and therapeutic challenge, in which ECP plays a major role.3,9
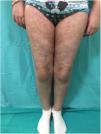
GVHD may present clinically in an acute or chronic form, independently of the number of days since transplant. The acute form (Fig. 1) is characterized by cutaneous involvement, which manifests as an erythematous micro and macro papular rash with mucosal lesions. A small percentage of patients may present erythroderma or blisters with Nikolsky sign. Extracutaneous manifestations such as hepatic and GI abnormalities may also be found. Staging ranges from 1 to 4 depending on clinical signs and symptoms and the percentage of body area involved. At the hepatic and GI level, it is modified based on serum levels of bilirubin and quantification of diarrhea, respectively.
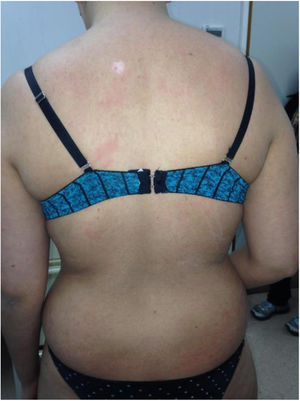
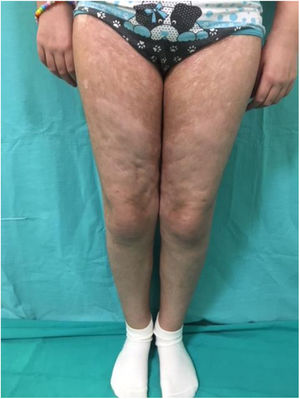
Chronic GVHD (Fig. 2) is a multisystemic disease and presents diverse clinical manifestations, similar to those observed in autoimmune syndromes, where cutaneous lesions may be lichenoid or sclerodermiform; the mucosa, GI system, liver, lungs and fascia and muscles may be involved; FM involvement may produce incapacitating physical sequelae. Severity is determined by the degree of involvement of each compromised organ (mild, moderate, and severe). The most commonly used scoring system is that of the National Institutes of Health (NIH), which describes severity based on the functional repercussion of the organs involved, and ranges from 0 to 3 for each organ.1,10,11
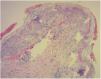
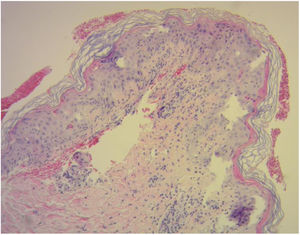
Diagnosis of cutaneous GVHD is based on the clinical examination and is confirmed by histopathology study. Histology reveals a lymphocytic infiltrate in the superficial dermis, cytopathic changes in the keratinocytes, which are surrounded by satellite lymphocytes. Four degrees of severity are recognized, based on the degree of dermal-epidermal involvement (Fig. 3).10,11
Standard first-line treatment of GVHD consists of corticosteroids (response to them is an important survival factor) and calcineurin inhibitors, whereas other immunosuppressive therapies are used in refractory disease. Despite these aggressive treatments, it is not possible to control GVHD in a considerable percentage of patients. There is therefore an urgent need to develop more selective strategies for managing GVHD, such as ECP.2–5
ECP is an immunomodulatory therapy based on leukapheresis, in which a system of apheresis is used to extract the mononuclear cells from the patient’s peripheral blood; these cells are then exposed to the effects of UVA light in the presence of a photosensitizing agent (8-methoxypsoralen), which induces apoptosis of leukocytes and generates an immune response.5,12,13
ECP in children is of considerable utility and the main difficulties involved are the type of venous access needed to maintain a good treatment dynamic, hemodynamic tolerance, and underlying hematologic disorders. Two methods exist for performing ECP: 1) the offline method, which is carried out in 2 stages (separation and photoactivation), in which the type of cell separator most commonly used in pediatrics is the COBE Spectra continuous-flow system, and 2) the online method, in a single stage, which has 2 cell-separation systems, a Therakos UVAR XTS™ discontinuous-flow system, and a CELLEX™ Therakos continuous-flow system.3,5,12
In their 2015 study, Kapadia et al14 showed that, in the pediatric population, the CELLEX™ system appears to be better tolerated than the UVAR-XTS™ system, as it reduces treatment time and hemodynamic complications. In our study, we use the UVAR-XTS™ system in 2 patients and the CELLEX™ system in the rest, due to the greater versatility of the latter system, as it can be used in double-needle mode in patients weighing less than 30 kg and with a low hematocrit. In this situation, double venous access is essential; in our study, half of patients required a central venous catheter. It should be noted that these catheters must be non-collapsible, double-needle, and with a diameter preferably of 8 F or greater. We also found that the shorter treatment time required by this device favored treatment tolerance in the children. With regard to the procedure, it should also be noted that the extracorporeal circuit must be treated with an anticoagulant; the choice and dose of anticoagulant are not standardized. The manufacturers recommend heparin and the protocol used is 250 U/kg diluted in 500 mL of normal saline solution, infused in a proportion ranging from 8:1 to 16:1, depending on the patient’s platelet count.
The ECP treatment plan and duration are also not standardized in pediatric patients with GVHD. Kanold et al 20074 recommend performing 3 sessions per week for both acute and chronic GVHD until maximum response is achieved and then, individualizing and gradually reducing frequency depending on the response. Halle et al 200215 in their series of chronic GVHD performed 2 sessions once per week for 2 weeks, and then every 2 weeks for 3 months, with gradual reduction in patients who showed improvement or stabilization. Uygun et al16 carried out the same regimen for acute and chronic GVHD of 2 sessions per week for 2 months, then every 2 weeks for 2 months, and finally, every month for at least 1 year. In our study, in acute GVHD, we performed 2 sessions per week for 4 weeks, with re-evaluation after 1 month and end of treatment at 3 months or at the time of maximum response, which was obtained in between 2 and 5 months (10 session). In chronic GVHD, we performed 2 sessions every 2 weeks, with re-evaluation after 3 months and end of treatment depending on response, with a duration that ranged between 3 and 41 months (10–90 sessions). It is important to note that end of treatment is defined in an interdisciplinary manner, generally after having reduced or suspended the immunosuppressants, individually in each patient.
Contraindications of ECP in pediatric patients are similar to those observed in adults: fever, sepsis, hemodynamic instability, and the recommendation is that patients should have a hematocrit of greater than 28%, a platelet count of over 20,000/mm3, and a neutrophil count of over 1000/mm3.3,17,18
No clinical trials of ECP in pediatric patients exist; studies are mostly case reports (Table 5).
Published Experience of ECP in Pediatric Population.
| Author/Reference | No. of Patients, a-GVHD/c-GVHD | Median age, y and IQR | Complete Response Rate | Technique Used | No. of Sessions | Survival |
|---|---|---|---|---|---|---|
| Kanold et al4 2007 | 27, 12/15 | 13.5, 4–18 | 7/12 (58%) in a-GVHD; 4/15 (26%) in c-GVHD | COBE Spectra and UV-MATIC | 24, 10–68 | 8/12 (67%) for a-GVHD and 10/15 (67%) for c-GVHD |
| Duzovali et al9 2006 | 7 c-GVHD | 10, 5–17 | 3/6 (skin), 1/5 (liver) | UVAR XTS (Therakos) | 21, 3–31 | 3/7 living |
| Halle et al15 2002 | 8 c-GVHD | 10, 5–15 | 3/8 (skin), 4/6 (liver), 5/5 (GI) | COBE Spectra and UV-MATIC | 28.5, 10–66 | 6/8 living |
| Uygun et al16 2015 | 12 (6/2 and 4 overlapping) | 12, 2–17 | 7/10 (70%) in a-GVHD; 4/6 (66%) in c-GVHD | Therakos CELLEX | 16, 4–36 | 8/12 living |
| Perotti et al19 2010 | 73, 50/23 | 9.9 (a-GVHD) 11.8 (c-GVHD) | 16/50 (32%) in a-GVHD; 5/23 (22%) in c-GVHD | COBE Spectra and UV-MATIC | 18 (12–24) in a-GVHD and 34 (16–43) in c-GVHD | 23% for a-GVHD and 19% for c-GVHD |
| Messina et al.20 2003 | 77, 33/44 | 8.6, 0.3–20.5 | 18/33 (54%) in a-GVHD; 15/44 (34%) in c-GVHD | UVAR XTS (Therakos) or COBE spectra | 8 cycles (2–20) in a-GVHD, in c-GVHD NR | 69% for a-GVHD and 77% for c-GVHD |
Abbreviations: a-GVHD, acute graft-vs-host disease; c-GVHD, chronic graft-vs-host disease.
In the studies reported in the literature, most patients presented a complete response in skin, unlike in our study, in which all patients except one developed a partial response. This may be due to the differences in the response-evaluation protocols. In our protocol, a complete response in skin was only reported if all cutaneous signs and symptoms improved completely and, of these, hyperpigmentation was what improved first, followed by sclerosis and induration. These favorable changes contributed to a notable recovery of the patients’ mobility. The response in other organs was similar to that reported in other published studies.
Of the more recent studies, in 2015, Uygun et al16 evaluated 12 patients treated with ECP. This group of patients had a similar number of cases to ours and the cutaneous responses were similar in both groups, although in our study, we obtained a better hepatic response. Other reports of series with few cases exist, such as those by Duzovali et al 20079 with 7 patients and Halle et al 200215 with 8, although with no patients with acute GVHD. When compared to our study, we obtained a better overall cutaneous and hepatic response.
In general, ECP was well tolerated, with few adverse effects. The literature reports fever, abdominal pain, episodes of transitory low blood pressure, and infections associated with the catheter.3,5,13,15,17 These last 2 adverse effects were the only ones observed in our study.
In conclusion, ECP is a very well-tolerated and safe treatment for GVHD in pediatric patients, with a high clinical response rate, mainly in the skin and mucosa, and which makes it possible to reduce the dosage of corticosteroids and other adjuvant immunosuppressive treatment.
Based on these observations and given the multiple adverse effects of the drugs used in this disease, it may be inferred that in pediatric patients with GVHD, ECP may be useful for reducing morbidity and mortality, and improving quality of life.
This is the first descriptive study of acute and chronic GVHD treated with ECP in a Latin-American pediatric population; we therefore believe that more studies in this area of research are needed, mainly to evaluate the impact of this treatment on the survival and quality of life of these patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cueto Sarmiento KY, Baquero Rey JA, Andrade Miranda A, Bruey SA, Makiya ML, Mazzuoccolo LD, Enz PA. Fotoféresis extracorpórea en enfermedad injerto contra huésped en una población pediátrica, ACTAS Dermo-Sifiliográficas, 2021;112:625–631.