Although national guidelines on biologic agents for treating moderate to severe psoriasis in adults have been published in several countries, increased knowledge on the practical aspects of their implementation is required.
ObjectiveThe objective of this study was to survey Spanish dermatologists to determine their expert opinions on practical aspects of psoriasis treatment with biologics.
Materials and methodsAn online survey was sent to 309 dermatologists who belong to the Spanish Psoriasis Group and/or the Spanish Academy of Dermatology and Venereology (AEDV). The questionnaire was designed specifically for the study and included items on various aspects of the treatment of psoriasis in clinical practice. Six coordinators in different geographic areas worked together to write the final expert report.
ResultsThe response rate was 97% (300 returned questionnaires). The biologics preferred, or considered to be the best option (median score 4 out of 4 points) by respondents, were infliximab for its short-term efficacy (74% of the respondents) and rapid onset of action (78%); ustekinumab for convenience of administration (73%); and etanercept because of its suitability for cyclic treatment (71%), safety in long-term use (72%), and the possibility of temporary interruption of treatment under certain circumstances (76%). Etanercept was assigned the highest evaluations for safety and expected survival time (scored 5 on each item by 49% and 33% of the respondents, respectively). Thirty percent of the respondents considered that clinical guidelines contain important information for therapeutic management of psoriasis.
ConclusionsThis study provides a unique perspective on the opinions of a large sample of dermatologists as regards current treatment of psoriasis with biologics in Spain.
Aunque se dispone de directrices nacionales sobre el tratamiento de la psoriasis moderada-grave del adulto con biológicos, es esenciar ampliar el conocimiento sobre aspectos prácticos en el uso de estos agentes.
ObjetivoEl objetivo de este estudio fue recoger la opinión de los dermatólogos españoles expertos en el manejo de la psoriasis sobre aspectos prácticos de su tratamiento con biológicos.
Material y MétodosEncuesta on-line remitida a 309 dermatólogos pertenecientes al Grupo Español de Psoriasis o miembros de la Academia Española de Dermatología. La encuesta diseñada específicamente para el estudio incluía preguntas sobre diferentes aspectos del tratamiento de la psoriasis en su práctica clínica. Seis coordinadores, representativos de las diferentes zonas geográficas, elaboraron el informe final de expertos.
ResultadosLa tasa de respuesta fue del 97% (N=300). Los biológicos considerados como opción preferida o más favorable por los encuestados (opción 4 de 4) fueron: infliximab por su eficacia a corto plazo (74%) y rapidez de acción (78%); ustekinumab por su conveniencia en la admi-nistración (73%); y etanercept por posibilidad de administrar en ciclos (71%), seguridad a largo plazo (72%) y posibilidad de discontinuar en situaciones especiales (76%). En cuanto a la percepción clínica de seguridad y “supervivencia”, otorgaron la máxima valoración (opción 5 de 5) a etanercept un 49% y 33% de los encuestados. Un 30% de los encuestados consideran muy relevantes las Guías de manejo terapéutico con biológicos.
ConclusionesLos resultados de este estudio proporcionan una perspectiva inédita sobre la opinión de una amplia muestra de dermatólogos españoles en España, respecto al uso actual de biológicos en el tratamiento e psoriasis.
Psoriasis, a chronic inflammatory skin disease that is often associated with arthritis and other conditions, affects 1.4% of the Spanish population.1 The alternatives available for treating psoriasis have multiplied in recent years with the introduction of biologic agents, which have no organ-specific toxic effects; as a result, these new therapies have transformed expectations.2 The choice of treatment must be tailored to the individual and based on current guidelines,3–9 but little is known about dermatologists’ preferences in routine practice. The main purpose of this study was to describe expert Spanish dermatologists’ preferences in prescribing biologic agents to manage moderate to severe psoriasis.
Material and MethodsStudy Population and SettingWe sent an online survey to 309 dermatologists who belong to the Spanish Psoriasis Group (n=89) and/or the Spanish Academy of Dermatology and Venereology (AEDV). The recipients of the survey were considered experts on the treatment of moderate to severe psoriasis and were prescribers of biologics.
Study DesignThe 7 study coordinators agreed on the design of a specific questionnaire that included some open-ended questions and topics for comment (Table 1), for which free-text fields were provided. Most items, however, were answered on a Likert-type scale for expressing preference. These 35 items referred to the sequencing of traditional systemic treatments, criteria governing the decision to start biologic therapy, the advantages of different biologics with respect to a range of attributes, the percentage of patients on continuous treatment, the clinical importance of registry-derived data on the risk of latent tuberculosis reactivation, perception of the safety and expected “survival” of the different biologics, the prevalence and management of joint disease and other comorbidities, and knowledge and perception of the importance of available management guidelines. The comments on open-ended items (written in free-text fields) were grouped by geographic areas (northeast, northwestern, southeastern, central, southern Spain, and the Balearic and Canary Islands) and then evaluated by 7 regional coordinators, who wrote reports organized by subject following a previously designed template. The group then wrote an expert report covering the entire Spanish territory.
Topics With Free-Text Fields for Open Comments.
| Topic 1: Current clinical management of psoriasis: traditional systemic and biologic therapies |
| 1. According to approved summaries of product characteristics for the different biologics available to treat moderate to severe plaque psoriasis, biologics are considered second-line treatments for use by patients who have contraindications for a standard systemic treatment (including ciclosporin, methotrexate, and psoralen – UV-A treatment) or who have not responded to such treatment. |
| 2. Experience in the management of patients with moderate to severe plaque psoriasis. |
| 3. Description of the use of standard systemic therapies before deciding to transition to a biologic agent: ideal sequencing and number of systemic therapies used before transitioning. |
| 4. Problematic circumstances seen most often when treating patients with psoriasis and that interfere with the use of each option proposed or that encourage transitioning away from a chosen option. |
| 5. Factors considered when deciding to transition a patient away from a standard systemic therapy to a biologic agent. |
| 6. What is the average time required to transition from a standard systemic to a biologic therapy in your experience? Do you think it would be a good idea to shorten this time in the interest of optimizing management of the patient's treatment? |
| 7. Advantages of biologic agents for management of psoriasis. What do you think is the ideal profile of a patient to be treated with each of the biologics proposed? |
| 8. Evaluate each biologic agent according to efficacy for reaching a PASI target by 12 weeks or by 24 weeks, or for maintaining a PASI response over a course of treatment. |
| 9. The most effective and safest treatments to combine with biologic agents. |
| Topic 2: Anti-TNF biologics: data from national and international registries |
| Data in national and international registries on the diverse indications for anti-TNF biologics indicate that these agents differ in terms of their associated risk of latent tuberculosis reactivation and their “survival” rates. According to these data, the fusion protein seems to be associated with lower risk of tuberculosis reactivation and longer survival. |
| 1. Importance placed on this information from registries. Is this information decisive when prescribing a biologic agent for psoriasis? |
| 2. Clinician's perception of the differential safety profile for the fusion protein in comparison with antibodies. |
| 3. Clinician's perception of the association of some TNF antagonists with longer survival (percentage of patients who remain in treatment with each drug after 1, 2, or 3 years). Personal experience; cases for comment. |
| Topic 3: Comorbidities |
| Psoriasis is considered a systemic inflammatory process associated with circulatory, joint, metabolic, and other diseases. |
| 1. Approach to treating the patient with psoriatic arthritis in dermatology. Personal experience; cases for comment. |
| 2. Importance and management of comorbidity in the patient with psoriasis. Personal experience; cases for comment. |
| Topic 4: Guidelines and consensus statements |
| Published guidelines are a point of reference, providing the dermatologist with a summary of the currently available evidence that can facilitate optimal treatment of a patient; they can also serve as a point of reference for hospital managers and health authorities. Importance of guidelines and adherence to them. Aspects that should be considered in new guidelines for the use of biologics in psoriasis. |
| Topic 5: Patient access |
| Name the main administrative roadblocks you face when prescribing biologic agents within the public health system |
Abbreviations: PASI, psoriasis area and severity index; TNF, tumor necrosis factor.
We calculated that a sample size of 300 would be required to keep the margin of error in estimation of percentages below 3.5% with a 95% level of confidence and an expected response rate of 70%. Descriptive statistics were prepared, and preferences were compared with the Fisher exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Subgroup analyses were made for age, sex, level of experience, and geographic area. Only statistically significant and important differences between subgroups are reported. Two-tailed tests of significance (α=.05) were used in all comparisons.
ResultsSociodemographic CharacteristicsNine of the 309 dermatologists we emailed did not respond. By geographic location the distribution was as follows: south, 61 (57 responded); central, 60 (56 responded); northwest, 60; northeast, 60; southeastern coastal, 48; Canary Islands, 13; and Balearic Islands, 17 (16 responded). Of the 300 respondents, 170 were men (56.7%) and 130 women. Ages ranged from 29 to 68 years (mean [SD], 43.4 [9.1] years; median [interquartile range], 43 [36–50] years). Men were older on average (mean, 45.2 [8.9] years) than women (41.1 [9.0] years) (P=.0001, t test). All were dermatologists, with experience in the specialty reflected by a median of 15 (8–22) years of practice. Men had a mean of 17.6 (8.9) years of experience in the specialty and women a mean of 14.0 (8.6) years (P=.0005, t test). Only 4 respondents worked mainly outside a national public health system hospital or primary care center.
The respondents reported attending a mean of 468 (186) patients each month (a mean of 55 [34] with psoriasis); the psoriasis is moderate to severe in 39% on average (median, 33.5% [20%–60%]). A median of 62% (30%–90%) of the patients with moderate to severe psoriasis are on a biologic or other systemic therapy. We observed great variation between geographic areas, with percentages ranging from 30% in the Balearic Islands to 81% in the Canary Islands (P=.001, ANOVA). No association with age, sex, or years of experience was evident.
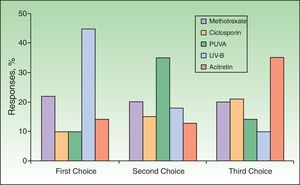
Use of Traditional Systemic Therapy and Transitioning to Biologic AgentsIn response to the question about the ideal sequencing of traditional systemic therapies before deciding to start biologic therapy, 45% named UV-B treatments as the first they prescribe, while 22% expressed a preference for starting with methotrexate. Acitretin, ciclosporin, and photochemotherapy (psoralen–UV-A [PUVA]) were named as first treatments by fewer than 15% of the respondents, mainly in relation to their poorer safety profile. The second-line therapy was PUVA for 35% and methotrexate for 20%. Acitretin was named as the third choice by 35%, ciclosporin by 21%, and methotrexate by 20% (Fig. 1).
Among male dermatologists, 14.6% reported prescribing only a single systemic treatment before starting a biologic agent; 56.7% prescribed 2 systemic therapies before switching, and 28.7% used more than 2. Among women 3.2% reported prescribing only a single systemic treatment before a biologic agent; 54.4% prescribed 2 systemic therapies before a biologic agent, and 42.4% used more than 2. These differences between men and women were statistically significant (P=.0012; χ2 test), but no association with geographic area or years of experience was detected.
The mean maximum weekly dose of methotrexate indicated by the respondents was 20 (5) mg. The median cumulative dose the respondents thought should not be exceeded was 2g (1.5–3g). The median maximum dosage of ciclosporin reported was 2 (1.5–3) mg/kg/d and the median maximum duration of treatment was 12 (6–24) months. The median maximum dose of acitretin was 50 (35–50) mg. Finally, the median limit on PUVA sessions was 60 (30–200).
When asked which factors were to be considered when deciding on switching from a traditional systemic therapy to a biologic agent, the largest percentage of respondents named toxicity and adverse effects as their main concerns (45%); efficacy was named by the next largest group (38%). Only 16% considered comorbidities when deciding to start biologic therapy. Seventy-three percent of the dermatologists found that 2 years or more passed before patients were switched from traditional to biologic therapies, and 66% felt that this transition period should be shorter.
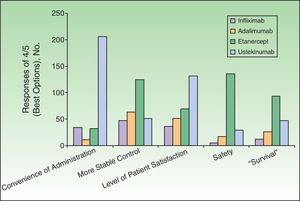
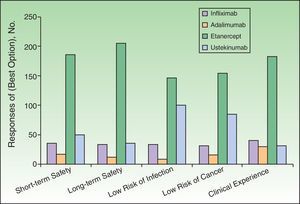
Dermatologists’ Perception of Biologic AgentsThe respondents assessed the different biologics with respect to several attributes, assigning scores on a scale of 1 to 4, where 1 was the least favorable assessment and 4 the most favorable (Table 2 and Figs. 2–4). The highest score on the different attributes was given to the following biologics: infliximab for rapid onset of action (78%) and short-term efficacy (74%); ustekinumab for mechanism of action (44%), immunogenicity (45%), convenience of administration (73%), and level of patient satisfaction (46%); and etanercept for sustained long-term efficacy (44%), short-term (66%) and long-term (72%) safety profile, clinical experience with the drug (65%), possibility of cyclic (rotational) therapy (71%), possibility of temporary interruption of treatment under certain circumstances such as infections or surgery (76%), efficacy on restarting treatment (65%), lower risk of infection (51%) or cancer (55%), possibility of combination regimens (55%), and stable control of disease (44%). Adalimumab did not receive the highest score for any attribute, but it was considered a second-line therapy by some dermatologists in percentages ranging from 33% to 69% for all attributes except the possibility of rotational regimens (ustekinumab was named by 38%), efficacy on restarting treatment (ustekinumab, 39%), and convenience of administration (etanercept, 55%).
Median Scores Assigned by Survey Respondents to Evaluate Biologic Agents According to Specific Attributes.a
| Attributes | Adalimumab | Etanercept | Infliximab | Ustekinumab |
| Mechanism of action | 2 | 3 | 2 | 3 |
| Development of neutralizing antibodies | 2 | 3 | 1 | 3 |
| Rapid onset of action | 3 | 2 | 4 | 2 |
| Short-term efficacy | 3 | 2 | 4 | 2 |
| Sustained long-term efficacy | 3 | 3 | 1 | 3 |
| Short-term safety profile | 3 | 4 | 1 | 2 |
| Long-term safety profile | 3 | 4 | 1 | 2 |
| Clinical experience | 3 | 4 | 2 | 1.5 |
| Possibility of rotational treatment regimens | 3 | 4 | 1 | 2 |
| Possibility of temporary interruption under certain circumstances (infections, surgery, etc.) | 3 | 4 | 2 | 1 |
| Efficacy on restarting therapy | 3 | 4 | 1 | 2 |
| Lower risk of infection, especially tuberculosis | 2 | 4 | 1 | 3 |
| Lower risk of cancer | 2 | 4 | 1 | 3 |
| Possibility of treatment combinations | 3 | 4 | 2 | 1 |
| Convenience of administration | 3 | 2 | 1 | 4 |
| More stable disease control | 3 | 3 | 1 | 2 |
| Level of patient satisfaction | 3 | 3 | 1 | 3 |
With respect to other therapies considered safe for combination with biologics, the respondents mentioned topical agents for limited, resistant plaques and seasonal exacerbation and acitretin for hyperkeratotic palmoplantar psoriasis in combination with methotrexate or UV-B phototherapy for the effective control of seasonal exacerbations. They also underlined the importance of avoiding the loss of response over time (especially with infliximab, or, on occasion, when transitioning between biologics).
Seventy-seven percent of the respondents judged that registry data on latent tuberculosis reactivation were of high (42%) or very high (35%) importance in accounting for the favorable profile of etanercept in comparison with tumor necrosis factor antagonists. Nevertheless, only 20% of the dermatologists considered such information to be a decisive factor in their decision to start biologic therapy given that psoriasis patients undergo systematic screening.
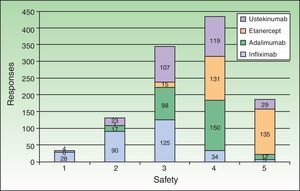
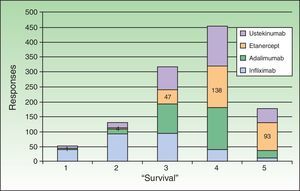
All the biologic agents except infliximab were considered generally safe or very safe (scoring 4 or 5 out of 5 points) by percentages of respondents ranging from 52% (ustekinumab) to 88% (adalimumab) (Fig. 4). Etanercept received the highest evaluation from the largest percentage of respondents (scored 5 out of 5 by 48%). This biologic was considered to be associated with lower risk of infection in general and particularly of latent tuberculosis reactivation (scored 4 or 5 out of 5 by 86%). That etanercept is the biologic with which we have accumulated the most experience in patients infected with hepatitis C virus or human immunodeficiency virus was a view expressed by some of the respondents in open text boxes; this opinion was based on their own clinical practice and the available literature. Concerning the concept of drug survival (defined as the duration of continuous treatment without need to withdraw the agent due to primary or secondary failure or adverse events), etanercept once again received the highest score (5 out of 5) from the largest percentage of respondents (33%) (Fig. 5). Etanercept was also the biologic that is routinely prescribed in intermittent courses by the largest group (19.2%), followed by adalimumab (11.6%), ustekinumab (7.6%), and infliximab (2.2%).
Managing Comorbidities in PsoriasisTwenty-eight percent of the surveyed dermatologists estimated that at least 10% of their psoriasis patients have joint involvement, and 60% estimated that figure to be between 10% and 20%. Thirty-seven percent reported routinely examining joints, and 13% said they prescribe therapy for psoriatic arthritis whereas the remaining respondents refer the patient to a rheumatologist for evaluation and treatment. The comorbidities perceived to be most frequent were endocrine disorders (median prevalence, 40% [30%–60%]) and psychological disorders (median prevalence, 40% [20%–60%]). Cardiovascular comorbidity (median prevalence, 20% [10%–30%]) was the next most common. Twenty-nine percent expressed the opinion that comorbidity was a very important factor (scored 5 out of 5) when they were considering transitioning from a traditional systemic to a biologic therapy. A small number of respondents (around 10%) reported prescribing drugs to treat conditions associated with psoriasis.
Guidelines for Managing Psoriasis TherapyFinally, 29% of the respondents expressed the belief that clinical guidelines play a very important role (scoring this concept 5 out of 5) in the management of patients. Spanish national guidelines were preferred by 42%, while 22% preferred international guidelines and 20% local recommendations.
Complementary Comments in Free-Text FieldsAccording to the respondents’ freely expressed opinions, biologic therapy should be maintained for 24 weeks before efficacy can be evaluated. If the psoriasis area and severity index has not improved by between 50% and 75% (PASI 50–75) by 24 weeks, most would prescribe a traditional systemic therapy in combination with the biologic therapy. A minority of the respondents would switch to another biologic agent. Most believed it is more important to achieve a safe, long-term response than a rapid one of short duration. When response is optimal (PASI 90 or better), many said they would choose to start intermittent therapeutic regimens or lengthen the intervals between doses. Etanercept was named as the biologic most often used in intermittent dosing regimens.
DiscussionSurveys of dermatologists’ perceptions of systemic therapies for moderate to severe psoriasis are scarce in the literature. Our search in PubMed for the terms survey AND dermatologist* AND psoriasis AND (treatment OR therapy) returned 176 titles on May 28, 2012. Yet only 9 studies similar to ours had been published in the previous 20 years,10–18 and only 4 of them had surveyed a large number of informants. One of these studies investigated the attitudes of 628 Belgian participants toward phototherapy and traditional systemic therapy.10 Another surveyed 531 members of the British Association of Dermatology on the topic of monitoring safety.11 A third, concerning adherence to guidelines, reported responses from 353 participants in the Netherlands.13 The fourth was a survey of 1000 dermatologists who were members of the US National Psoriasis Foundation or who treated psoriasis patients.18 In some of these studies, the response rates were very low, ranging from 39%18 to 49%,10 although the British survey, with a response rate of 71% was an exception.11
Our study has profiled Spanish specialists in psoriasis management, providing information on their psoriasis caseload, their perceptions of traditional systemic and biologic therapies, and their prescribing preferences. Men make up the largest proportion of this population (57%), in which the average age was 43 years at the time of the survey and the average number of years of experience in the specialty was 15. The mean age and length of experience of women were significantly lower. The immense majority of respondents work mainly in the public health system and in a hospital. They attend 23 patients daily on average. A mean of 11% (median 10% [6%–13%]) of their patients are being treated for psoriasis, which is moderate to severe in almost 40% of cases on average (or 4.4% of the total caseload). This percentage is comparable, though somewhat lower, than the figure of 6.8% reported in another Spanish survey of 164 specialists in this disease.15 A survey of dermatologists working in nonhospital settings in the United States (in the state of Ohio) found that patients with psoriasis of any degree of severity accounted for 4% of the caseload16; that level confirms that the Spanish dermatologists participating in this survey are especially dedicated to forms of the disease requiring systemic therapy.
The percentages of patients with moderate to severe psoriasis who are treated with traditional systemic therapy or biologic agents fall between 61% and 73% in most areas of Spain, with the exception of the Canary (81%) and Balearic (30%) Islands. The reasons for differences in the 2 Spanish island communities may merit more detailed study, although bias may have affected our findings for areas with small numbers of participants who had different preference profiles. For example, whereas 9 out of 13 Spanish specialists use biologics to treat 90% or more of their patients with moderate to severe psoriasis in the total population of respondents, in the Balearic Islands 3 out of 7 specialists prescribe biologics for 10% or fewer of their patients with this level of disease. We did not differentiate between traditional and biologic systemic therapies in this survey, but our findings are consistent with those from another Spanish survey which found that 45.8% of patients were prescribed a traditional systemic and 22.9% a biologic therapy. Our respondents’ preferences with respect to nonbiologic treatments were first for phototherapy and photochemotherapy, although we observed differences by geographic area: the preference for these therapies is less marked in the south and in the Canary Islands, possibly because of more abundant sunlight in those regions or the relative scarcity of phototherapy units.
Most respondents (85%) reported using 2 or more traditional systemic therapies before transitioning to a biologic, although female dermatologists use more than male specialists. The maximum weekly, daily, or cumulative doses of the various systemic therapies do not differ from those published in guidelines, although we note that the maximum cumulative dose of methotrexate (2g) and the duration of treatment with ciclosporin (1 year) the respondents used on average can be considered conservative. The decision to start treatment with a biologic agent is more often based on concerns for toxicity and safety than on efficacy. One out of every 6 respondents (16%) consider that the potential effect of a biologic agent on concomitant conditions is a very important aspect to consider when changing a patient's therapeutic regimen. Two years or more pass before 73% of patients are transitioned to a biologic therapy, meaning that these patients have probably reached the maximum cumulative dose for the previous treatment. Two out of every 3 of the dermatologists we surveyed, however, think that patients should make (or be allowed to make) the decision to change earlier.
The respondents’ assessments of the different biologics probably reflect their personal experience of reading the literature, attending conferences, etc. Even though the response after a patient has been on a biologic therapy for 3 to 4 months is highly important, these dermatologists generally wait 24 weeks to assess efficacy and decide whether to increase the dosage (the step chosen in most cases if treatment failure is observed), add a traditional systemic treatment, or switch to another biologic. Comments in the free-text fields expressed a tendency to place more importance on a safe long-term response than on a rapid one of short duration. Furthermore, when a patient's response is optimal many prescribers choose to lengthen the intervals between doses or use an intermittent dosing regimen. Etanercept is the biologic most often used in such intermittent regimens. Our respondents are aware of the rapid onset of action of infliximab and the convenience for the patient that ustekinumab offers, but etanercept seems to enjoy their highest regard overall, and adalimumab also has a generally favorable profile. Etanercept is perceived by the majority to be the safest biologic, although all these agents are considered generally safe or very safe. Data on cases in rheumatology registries with respect to the reactivation of latent tuberculosis and other infections are considered important but hardly determining factors when deciding to start psoriasis patients on biologic therapy. This attitude may derive from the possibly lower degree of iatrogenic immunosuppression in these patients,19 which is also reflected in a comparison of rates of serious adverse effects in case series in which adalimumab has been used for different indications.20
The survival of the various biologics (or patient adherence to treatment) is considered good in most cases, with scarce differences between them. When the respondents assigned scores from 1 to 5, the means and medians, respectively, corresponding to each agent were as follows: infliximab, 2.6 and 3; adalimumab, 3.6 and 4; ustekinumab, 3.7 and 4; and etanercept 4.1 and 4. In free-text field comments, some respondents expressed the opinion that the longer survival attributed to etanercept is related to the absence of neutralizing antibodies, which would explain the sustained long-term response perceived.
The respondents’ beliefs about the prevalence of comorbidities cannot be compared with actual figures for Spain because studies are not available for the entire territory. However, their perception of the prevalence of cardiovascular disease in patients with psoriasis (of any level of severity) is on average consistent with figures for Germany, where 12.1% have diabetes and 35.6% have hypertension.21 In any case, 29% of these dermatologists believe that comorbidities are very important factors to consider when deciding to transition to a biologic, although this consideration is a determining factor for fewer (16%). Freely expressed comments mentioned that because many psoriasis patients are overweight, it is important to intervene in dietary habits to encourage the weight loss that will improve response to biologic therapy. The presence or absence of psoriatic arthritis is also thought to be a key factor in choosing a therapy. When joint disease is present, most of the dermatologists choose to use etanercept or adalimumab, with infliximab ranking third. Although the respondents stated that available guidelines or other directives are important (expressing a preference for Spanish publications), only 29% said they consider them to be highly important (scoring them 5 out of 5).
In conclusion, this survey of a large sample of Spanish dermatologists who specialize in the management of moderate to severe psoriasis provides new perspectives on these physicians and their prescribing behaviors and on their evaluation of the various biologic agents currently available.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data protectionThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingThis study was promoted by the Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV). Members of the group did not intervene in the collection or interpretation of data or in the preparation of the manuscript. The authors did not receive fees for their contribution to the writing or publication of the manuscript.
Conflicts of InterestLluis Puig, Pablo de la Cueva, and Manuel Velasco have participated in clinical trials funded by Pfizer España and have received consultancy and speaking fees for activities funded by Abbott, Merck, Janssen, and Pzifer. Mario Linares and Ander Zulaica have participated in clinical trials funded by Pfizer España and have received fees for speaking at events sponsored by Pzifer España.
David Vidal has participated in clinical trials funded by Pfizer España and Abbott. José Suárez has participated in clinical trials sponsored by Pfizer España.
Carmen García Calvo works in the medical department of Pfizer España.
Please cite this article as: Puig L, et al. Informe de expertos en psoriasis: opinión de los dermatólogos espãnoles sobre el manejo de la psoriasis moderada-grave con agentes biológicos en pacientes adultos. Actas Dermosifiliogr. 2013;104:400-8.