The ex vivo confocal microscope is an imaging system designed to analyze freshly excised tissue using two diode lasers with different wavelengths. The technique can dramatically reduce margin analysis times and offers a sensitivity of 88% and a specificity of 89% relative to histopathology. A new technology has recently been developed that produces images more quickly and with a higher resolution than before. By means of a fusion mode that combines simultaneously scanned fluorescence and reflectance images, it produces digitally stained images that simulate the effect of hematoxylin-eosin staining. Application of this new technology has opened the door to real-time tissue diagnostics.
La microscopia confocal ex vivo es un sistema de procesamiento de tejidos que permite el análisis histológico inmediato de tejidos extirpados por medio de láseres diodo con distintos espectros de longitud de onda. En estudios previos la MCev reduce el tiempo de análisis de márgenes de forma muy notable, con una sensibilidad y especificidad respecto a la histopatología del 88% y 99% respectivamente. Recientemente se ha desarrollado una nueva tecnología que es capaz de proveer imágenes digitales con mayor velocidad y mejor resolución que las obtenidas en los dispositivos previos. Mediante el método de fusión (láseres de fluorescencia y reflectancia que escanean simultáneamente), se reproduce con una tinción digital la hematoxilina y eosina de forma inmediata en cada imagen. La implementación de esta nueva tecnología ha abierto definitivamente una puerta en el diagnóstico inmediato de tejidos.
Confocal microscopy is an imaging technique that makes it possible to view tissues in real time in vivo (ivCM) and ex vivo (evCM). The technique is based on the use of laser light, which passes through a system of lenses and shows the microscope image of the lesion in real time on an adjacent monitor by using specific software; it allows for diagnosis of lesions with cellular resolution from 0.5 to 1.0 μm.
Basic differences exist between ivCM and evCM techniques. The former is a noninvasive technique, in which all tissues are imaged on a horizontal plane with a single 830- nm diode laser light source. This imaging plane provides horizontal slices parallel to the skin surface, with a maximum penetration depth of 250 μm.
The images are obtained due to natural contrasts of molecules such as melanin and keratin, which refract intrinsically. ivCM can be used to facilitate diagnosis of suspicious lesions, to select the best site for obtaining tissue in a biopsy, or for preoperative outlining of surgical margins in lesions with poorly demarcated edges (e.g., lentigo maligna).1–3
evCM requires excision and scanning of the lesion. The excised tissue can be observed on a horizontal plane, as with ivCM, and on a vertical plane, which allows for correlation with the corresponding histopathology slide. Current evCM devices use 2 laser light sources with different wavelengths: 488 nm and 785 nm, corresponding to laser fluorescence and reflectance, respectively.
In an initial study of evCM in 2001, Rajadhyaksha et al, under the medical direction of Gónzalez,4 described the first images obtained from basal cell carcinoma excised using Mohs micrographic surgery. That study contains the first mention of the need to perform a staining technique to obtain better-quality images; it uses 10% acetic acid as a method to contrast the presence of nuclei with the surrounding tissue.
The use of this technique to find residual tumor tissue in basal cell carcinoma and squamous cell carcinoma by means of Mohs micrographic surgery was demonstrated in 2004.5 However, it was not until 20096,7 that acridine orange was introduced as a nuclear contrast agent, allowing for excellent contrast between the tumor, which is bright, and the surrounding tissue, which has a darker appearance.
Since then, advances have been made with that technique, and it has been shown to be able to reduce operating time for Mohs surgery by up to one-third and reliably replacing the use of the cryostat.8 At the same time, criteria have been described for differentiating basal cell carcinoma and squamous cell carcinoma from other adjacent structures.9–13
Studies carried out on different types of skin tumor have confirmed the superiority of evCM in evaluating surgical margins in real time and without the need for greater infrastructure, making it an efficient tool in clinical practice where this technology is available. It also makes it possible to determine tumor persistence after destructive procedures such as laser treatment,14 and to find residual tumor tissue in squamous cell carcinoma13 and tumors of the nail apparatus.15
To date, the most widely studied indication is use during Mohs micrographic surgery. In this context, evCM is of proven immediate clinical applicability and, in our experience, reduces operating times by at least two-thirds, with a sensitivity and specificity of 88% and 99%, respectively, and a positive predictive value of 98% and a negative predictive value of 97%.8
The application of this technique goes far beyond applications in dermatologic surgery and applications in inflammatory pathology have recently been published.16–18 Outside the field of dermatology, findings with evCM in breast, brain, lung, thyroid, bowel, lymph-node, esophagus, and oral-mucosa, and other tumors have recently been published.19,20
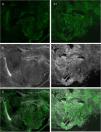
EquipmentThe VivaScope 2500® (Lucid Inc., Rochester, NY, USA) was one of the first devices used to obtain images in evCM for clinical use. This device had 1 fluorescence laser (488 nm) and 2 reflectance lasers (830 nm and 658 nm), and scanned the image with each of the lasers, thus increasing the final time of each examination. Over the past 2 years, a new device has been developed. This is the 4th generation VivaScope® (MAVIG GmbH, Munich, Germany), which has a higher resolution in tissue imaging and, for the first time, combines reflectance (with a785-nm laser) and fluorescence (488 nm) in the same image (Fig. 1). This makes it possible to simultaneously obtain fusion images, which combine fluorescence and reflectance to generate 2-color images, similar to conventional hematoxylin–eosin images (Appendix B, Video 1 in additional material).21,22 These images can be analyzed in each mode, separately, or combined; when combined, this is known as the fusion technique (Fig. 2). This device provides high-resolution images of the tissue between 3 and 7 minutes after initiating the scan and allows the images to be studied in the operating room and in the pathology laboratory simultaneously by means of a server that connects both terminals.
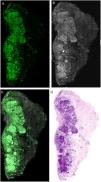
Preparation, Staining, and Scanning of the SampleThe tissue obtained from the complete excision or partial biopsy of a lesion is rinsed in saline solution to prevent image artefacts caused by blood. The tissue is then submerged in a 1 mmol/L solution of acridine orange for 20 seconds, is rinsed in abundant saline solution to remove excess stain, submerged in a 50% solution of acetic acid for 20 second, and, finally rinsed again in saline solution (Fig. 3). Larger tissue samples require longer contact with the acridine orange staining solution, as the staining would otherwise be insufficient to be able to image the staining of the nuclei in 100% of the sample. In compact tumors, 20 seconds are normally required to obtain real staining of the entire sample, due to the fact that the nuclei of some tumors, such as those of melanocytic cells, show difficulty in adequately taking the stain with acridine orange.
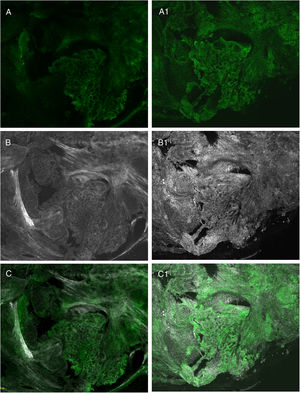
Recent studies have shown that the use of a combined technique of different stains, such as acetic acid and acridine orange, and the use of fusion images make it possible to further increase the quality of the final image by increasing the sharpness of the structures and allowing for imaging of characteristics that had not yet been described21,23 (Fig. 4).
Comparison of different stains: A, fluorescence with acridine orange; A1, fluorescence with acridine orange and acetic acid; B, reflectance with acridine orange; B1, reflectance with acridine orange and acetic acid; C, fusion with acridine orange; C1, fusion with acridine orange and acetic acid.
After the staining process has been completed, the tissue sample is placed on a slide and a slide cover is applied that, with the use of silica glue, modelling clay, or new devices developed for this purpose (J.P., J.M.), make it possible to firmly fix the sample prior to scanning (Fig. 5). When applying the slide cover, special care must be taken to remove any air bubbles by applying uniform pressure to the ensure surface to be scanned, thus allowing for complete images of the epidermis, dermis and hypodermis. Before beginning to scan images with the VivaScope 2500 4 th Gen® (MAVIG GmbH, Munich, Germany), a sufficient quantity of ultrasound gel must be applied to the lens that will transmit the laser light. At this moment, the operator, technician, or even the surgeon, will determine the zero point of the scanner. This point refers to the depth at which the tissue structures can be focused on for maximum sharpness and uniformity of tone, before starting the scanning process. The intensity of each laser is adjusted to obtain the appropriate luminosity and uniformity of the final image.
Finally, the selected scanned tissue is imaged by the fluorescence, reflectance or fusion laser, or by means of digital staining. This scanning process may be stopped at any moment to reposition the tissue, and can be restarted on multiple occasions without damaging the tissue.
In summary, the basic steps to obtaining a correct imaging process are the following: appropriate tissue staining, firm and uniform fixing of the sample on the slide in order to allow 100% of the area to be scanned, and identification of the appropriate zero point.
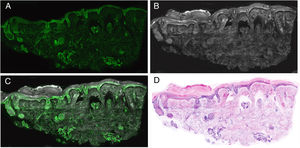
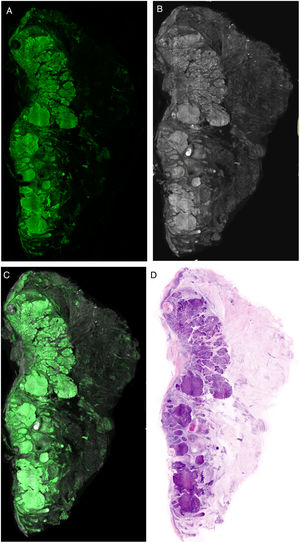
Interpretation of the ImagesThe resulting images show different characteristics depending on the laser used, providing essential keys for diagnosis. When the image obtained in fluorescence mode is observed, it will essentially show nuclear characteristics, as well as some refractile dermal elements, such as elastic fibers (Fig. 6A). The cytoplasm and most of the dermis are less evident. In reflectance mode, however, collagen fibers and the peritumoral stroma are seen more clearly (Fig. 6B). The morphological characteristics observed, for example, in basal cell carcinomas correlate to the same changes observed in conventional histopathology preparations (hematoxylin–eosin) (Fig. 6D). Thus, characteristics such as cell palisading, peritumoral clefting, and stromal reaction, and changes in the nuclei, an increased nucleus/cytoplasm ration, and cellular pleomorphism can be observed in a similar manner to digital hematoxylin–eosin staining. Moreover, different recently reported algorithms for processing digitally stained images help to improve the quality of tones and interpretation of the images.22 If we consider the analysis of the images under the different lasers, the reflectance laser will show characteristics that are not observed under the fluorescence laser. This is why the fusion images enrich the overall interpretation not only of tumor structure but also of stromal and peritumoral changes21,23 (Fig. 6).
Furthermore, in studies where tumor structures are not observed, it is important to determine whether the entire sample has been scanned to be really sure that we have a true negative.
In conclusion, the use of evCM with the VivaScope 2500 4th Gen® (MAVIG GmbH, Munich, Germany) opens up a wide range of potential applications, both in dermatology and in pathology in general, and represents a major transformation in the way tissues have been processed for the past 100 years. This technology reduces the process of obtaining images similar to those obtained from tissues in paraffin to a few minutes, thus simplifying the morphological study of the tissues and making the process more applicable to routine clinical and surgical practice.
FundingThis study was funded by the European Commission under the 7th Framework Programme, Diagnoptics.
Conflicts of InterestThe first 2 authors take part in research with MAVIG®.
The authors would like to thank the entire dermatologic surgery team at Hospital Clínic, Barcelona, Spain.
Please cite this article as: Pérez-Anker J, Malvehy J, Moreno-Ramírez D, Microscopia confocal ex vivo con método de fusión y tinción digital: cam-biando paradigmas en el diagnóstico histológico. Actas Dermosifiliogr. 2020;111:236–242.