Electrochemotherapy is a therapeutic option for the treatment of cutaneous and subcutaneous metastases from melanoma and other tumors. The procedure consists of the administration of anticancer drugs followed by locally applied electrical impulses to achieve an effect known as electroporation, which facilitates entry into the cytosol of drugs that cannot cross the cell membrane. The aim of this review is to evaluate the evidence that supports the use of electrochemotherapy as a therapeutic strategy in melanoma. We conducted a qualitative systematic review of the literature using advanced searches of bibliographic databases and full text reviews. Seven studies (3 systematic reviews and 4 cases series) were selected. The quality of the evidence was not good, but the coincidence of results for certain variables supports their consistency. Results of the meta-analyses favored electrochemotherapy over chemotherapy. Electrochemotherapy appears to be an effective procedure for the local treatment of malignant tumor nodules (evidence of intermediate or low quality). This inexpensive method is simple to apply, well tolerated, and achieves objective responses under certain circumstances. There is no evidence that electrochemotherapy alters the natural course of the disease and it should therefore be considered a palliative treatment. With an evidence level of 1- (minus), electrochemotherapy can be recommended for the palliative treatment of unresectable, locoregionally advanced melanoma (grade B recommendation).
La electroquimioterapia (EQT) es una modalidad de tratamiento de lesiones cutáneas y subcutáneas originadas por melanoma u otros tumores. El procedimiento consiste en la administración de agentes antineoplásicos, seguido de impulsos eléctricos locales, para conseguir un efecto conocido como electroporación, que permite la entrada al citosol de medicamentos que no difunden a través de la membrana celular. El objetivo de esta revisión es establecer la evidencia que sustenta la incorporación de la EQT como estrategia terapéutica en el melanoma.
Además, se ha llevado a cabo una revisión sistemática de la literatura con síntesis cualitativa. Se ha realizado una búsqueda cualificada de la literatura en bases de datos referenciales y a texto completo. Fueron seleccionados 7 estudios: 3 revisiones sistemáticas y 4 series de casos.
La calidad de la evidencia encontrada no es buena, pero la coincidencia de sus resultados en algunas las variables le da consistencia. Los metaanálisis muestran resultados a favor de la EQT frente a la quimioterapia.
La EQT parece un procedimiento efectivo en el tratamiento local de nódulos tumorales malignos (nivel medio o bajo de calidad de la evidencia). Es un tratamiento fácil de administrar, económico y bien tolerado con el que se consigue respuesta objetiva en circunstancias determinadas. No hay evidencia de que pueda afectar el curso natural de la enfermedad, por lo que debe considerarse un tratamiento paliativo.
Con un nivel de la evidencia 1–(1 menos), puede recomendarse la incorporación de la EQT para el tratamiento paliativo del melanoma locorregionalmente avanzado irresecable (fuerza de la recomendación: B).
Changes in the epidemiology of cutaneous melanoma have followed a characteristic pattern in Spain. In the last 30 years of the twentieth century, there was an acceleration in the increase, from 0.3 and 0.2 cases per 100 000 men and women, respectively, in 1972 to 3 and 3.8 cases, respectively, in 19921 then to 5.85 and 7.50, respectively, in 1998.2 Subsequently, the curve appears to reach a plateau, with incidence rates of 6.6 and 7.2, respectively, according to the GLOBOCAN source in 2012 and an overall mortality of 1/100 000 inhabitants.3
Although not the most frequent tumor nor responsible for most deaths, management of advanced cases of advanced melanoma is complex. The current standard treatment of both isolated and multiple tumor nodules is surgical excision, isolated limb perfusion, or radiotherapy. Some therapeutic alternatives in the treatment of metastatic lesions include cryotherapy, laser ablation, and radiofrequency ablation, with the choice of technique varying according to the nature of the lesion. After appropriate treatment, local recurrence of the tumor is hard to manage.4
Electrochemotherapy5,6 is an emerging treatment modality for cutaneous and subcutaneous lesions originating from melanoma or other primary and metastatic tumors. The procedure consists of intravenous or intratumoral administration of cytotoxic agents (bleomycin and cisplatin) followed by short and intense local electric pulses to achieve an effect called electroporation or electropermeabilization. The local application of electric pulses of a certain intensity, amplitude, and frequency temporarily destabilizes the cell membrane to allow transient and reversible formation of nonselective pores with a size of about 1 Angström without any impact on cell viability, thereby increasing permeability. Exposure of the cell membrane to electric pulses may lead to cell necrosis due to irreversible changes, whereas application of intense but short electrical pulses induces reversible electroporation, maintaining its viability. After application, the cytotoxic drugs that, given their structure and molecular weight would not easily diffuse across the cell membrane, can move from the intercellular space to the cytosol, resulting in increased in cytotoxicity at a lower dose.
The aim of this review is to evaluate the evidence supporting the use of electrochemotherapy as a therapeutic strategy for the treatment of unresectable, locoregionally advanced cutaneous melanoma.
MethodologyIn this systematic review of the literature with qualitative synthesis, an online search was undertaken of the literature in the reference databases Embase, Medline, and Expanded Science Citation Index, as well as the Cochrane Library and Centre for Research and Dissemination (CRD). The search was performed during February 2015.
The articles were selected independently by 2 investigators. The overall assessment of the evidence for each outcome variable was made by reviewing the study design, internal validity, consistency, and precision of the results, as well as other factors such as possible publication bias. For classification of evidence and recommendation grades, the Scottish Intercollegiate Guidelines Network (SIGN) classification was used.7 The Assessing Methodological Quality of Systematic Reviews (AMSTART) checklist was also applied.8
The predefined inclusion and exclusion criteria for selection of abstracts are shown in Table 1. The full text of the articles that met the inclusion criteria for title and abstract was reviewed independently by 2 investigators.
Inclusion and Exclusion Criteria for Article Selection (Using Abstracts) and Research Question in the PICO Format.
| Inclusion criteria | |
| Types of study | Clinical trials, cohort studies, SLR, meta-analyses that assess one of the outcomes included in the research question. In the event that no studies with these criteria were retrieved, the inclusion of nonanalytic studies and case-control studies was considered. |
| Languages | English, Spanish, French, and German |
| Other conditions | Technology assessment reports that analyzed details on the search strategy and methodological assessment of the studies were included. Agency assessment reports had to meet the checklist drawn up by the expert group in the 2001 INAHTA meeting. |
| Exclusion criteria | |
| Types of study | Animal or in vitro experiments, narrative reviews, series, case-control studies published prior to studies of higher evidence level. Studies of any design that had not assessed the variables specified in the research question or whose publication date was prior to the year 2000. |
| The PICO format of the research question | |
|---|---|
| Population | Patients aged over 18 years with: unresectable cutaneous and/or subcutaneous metastatic melanoma (in transit, satellite, distant) at any site. TNM stages IIIB (N2c), IIIC (N2c and N3), and IV (M1a for cutaneous-subcutaneous metastasis) Unresectable primary cutaneous melanoma (involvement of vessels, nerves, or other structures) or resectable only with mutilating surgery Patients with visceral metastasis and those receiving palliative treatment, to improve quality of life. |
| Intervention | Electrochemotherapy as monotherapy and as therapy combined with isolated limb perfusion in patients with high tumor load |
| Comparator | There are 3 therapeutic strategies for unresectable melanomas: Hyperthermic isolated limb perfusion Intralesional administration of interleukin 2, bleomycin, or perilesional administration of GM-CSF Locoregional radiotherapy Systemic treatment: chemotherapy, biochemotherapy, immunotherapy |
| Outcome | Effectiveness Clinical response can be measured as complete response (CR), partial response (PR), disease-free period (DFP or similar outcomes), overall survival (OS), recurrence rate (RR), stable disease (SD) Safety Adverse effects associated with the procedure Local or regional toxicity or systemic toxicity |
In this study, we used the criteria for unresectability and TNM corresponding to locally advanced melanoma according to the American Joint Committee of Cancer (AJCC) classification.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; INAHTA, International Network of Agencies of Health Technology Assessment; PICO, population (P), intervention (I), comparison (C) and outcome(s) (O); SLR, systematic literature review.
The research question posed was as follows: Is electrochemotherapy effective and safe for the treatment of unresectable melanoma (primary and metastatic) at any site in adults? This question was analyzed and transferred to the Population Intervention Comparator Outcome (PICO) format (Table 1).
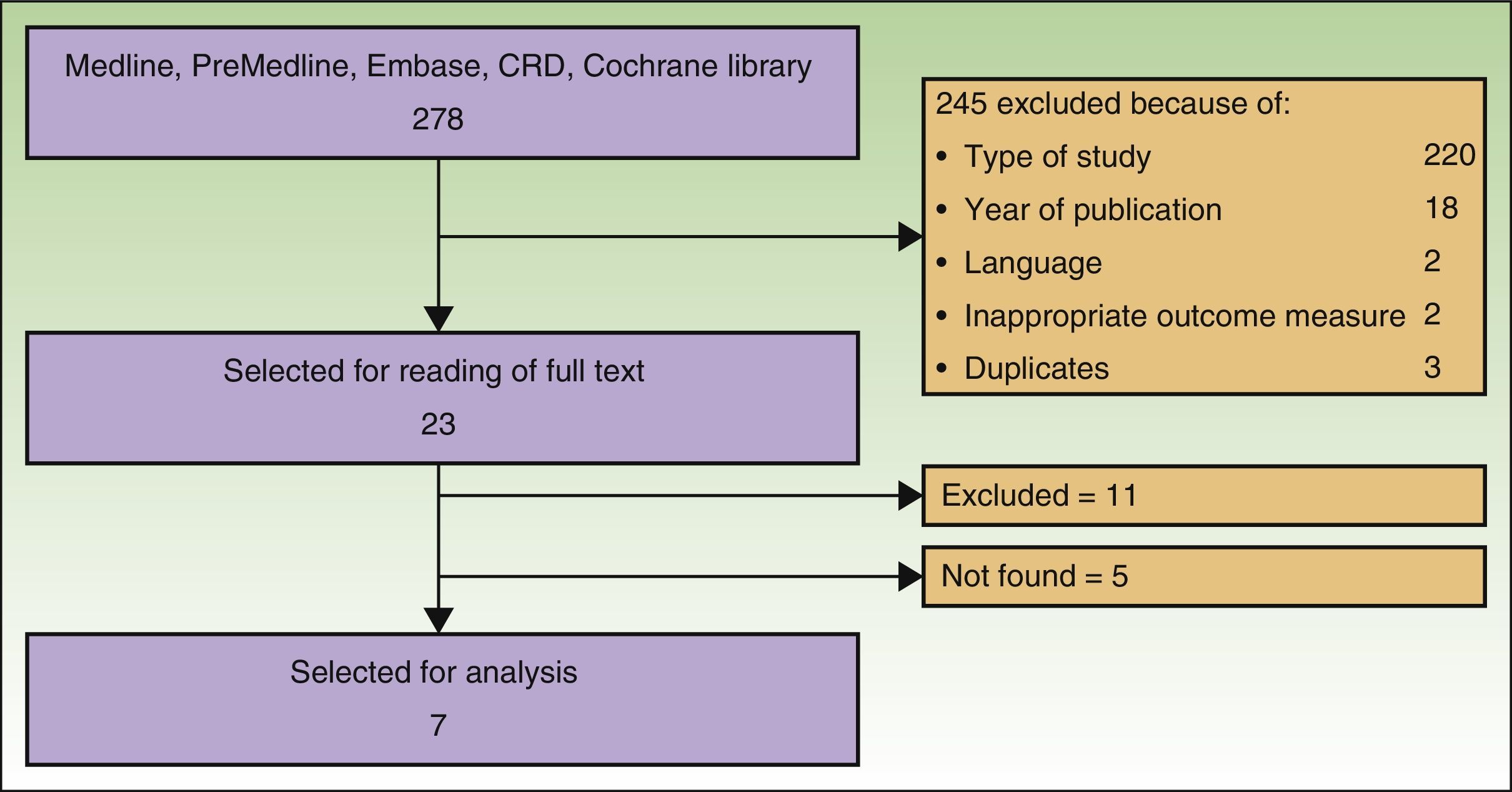
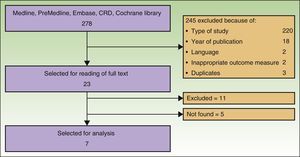
ResultsSearch ResultsAfter checking titles and abstracts, and applying the aforementioned criteria, 37 articles were selected to read the full text. Seven studies met the inclusion criteria, were considered appropriate and relevant, and were analyzed for this review. The flow diagram for article handling and selection is shown in Figure 1. References to studies were coded to facilitate their handling in the drafting of the manuscript: Spratt_2014,9 AETS_2011,4 Mali_2013,10 Caraco_2013,11 Riccoti_2013,12 Solari_2014,13 and Skarlatos_2011.14
As no clinical trials or cohort studies have analyzed the effectiveness of electrochemotherapy, we decided to include case series not included in systematic reviews or in the evaluation reports selected for assessment.
The search returned a National Institute for Health and Care Excellence (NICE) report from 2013.15 Although this report was a procedure guideline and so did not meet our inclusion criteria, it was nevertheless used for drafting the present article. Six studies were included in the meta-analysis of Spratt_2014. The remaining studies were excluded because of language of the report or type of study. Five articles could not be located.
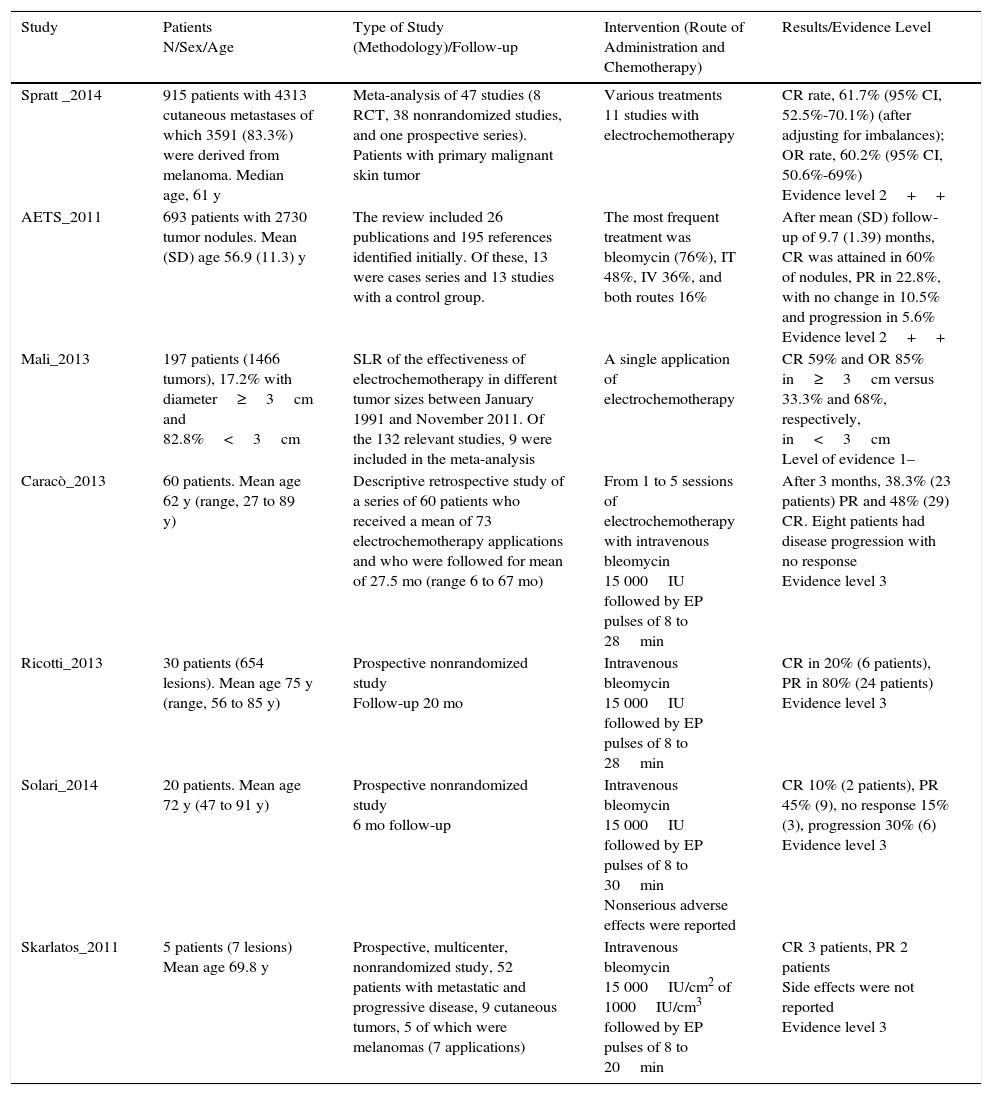
Study ResultsTable 2 summarizes the most relevant data for the studies analyzed and their evidence level.
Table Summarizing Evidence for Articles Analyzed.
| Study | Patients N/Sex/Age | Type of Study (Methodology)/Follow-up | Intervention (Route of Administration and Chemotherapy) | Results/Evidence Level |
|---|---|---|---|---|
| Spratt _2014 | 915 patients with 4313 cutaneous metastases of which 3591 (83.3%) were derived from melanoma. Median age, 61 y | Meta-analysis of 47 studies (8 RCT, 38 nonrandomized studies, and one prospective series). Patients with primary malignant skin tumor | Various treatments 11 studies with electrochemotherapy | CR rate, 61.7% (95% CI, 52.5%-70.1%) (after adjusting for imbalances); OR rate, 60.2% (95% CI, 50.6%-69%) Evidence level 2++ |
| AETS_2011 | 693 patients with 2730 tumor nodules. Mean (SD) age 56.9 (11.3) y | The review included 26 publications and 195 references identified initially. Of these, 13 were cases series and 13 studies with a control group. | The most frequent treatment was bleomycin (76%), IT 48%, IV 36%, and both routes 16% | After mean (SD) follow-up of 9.7 (1.39) months, CR was attained in 60% of nodules, PR in 22.8%, with no change in 10.5% and progression in 5.6% Evidence level 2++ |
| Mali_2013 | 197 patients (1466 tumors), 17.2% with diameter≥3cm and 82.8%<3cm | SLR of the effectiveness of electrochemotherapy in different tumor sizes between January 1991 and November 2011. Of the 132 relevant studies, 9 were included in the meta-analysis | A single application of electrochemotherapy | CR 59% and OR 85% in≥3cm versus 33.3% and 68%, respectively, in<3cm Level of evidence 1– |
| Caracò_2013 | 60 patients. Mean age 62 y (range, 27 to 89 y) | Descriptive retrospective study of a series of 60 patients who received a mean of 73 electrochemotherapy applications and who were followed for mean of 27.5 mo (range 6 to 67 mo) | From 1 to 5 sessions of electrochemotherapy with intravenous bleomycin 15 000IU followed by EP pulses of 8 to 28min | After 3 months, 38.3% (23 patients) PR and 48% (29) CR. Eight patients had disease progression with no response Evidence level 3 |
| Ricotti_2013 | 30 patients (654 lesions). Mean age 75 y (range, 56 to 85 y) | Prospective nonrandomized study Follow-up 20 mo | Intravenous bleomycin 15 000IU followed by EP pulses of 8 to 28min | CR in 20% (6 patients), PR in 80% (24 patients) Evidence level 3 |
| Solari_2014 | 20 patients. Mean age 72 y (47 to 91 y) | Prospective nonrandomized study 6 mo follow-up | Intravenous bleomycin 15 000IU followed by EP pulses of 8 to 30min Nonserious adverse effects were reported | CR 10% (2 patients), PR 45% (9), no response 15% (3), progression 30% (6) Evidence level 3 |
| Skarlatos_2011 | 5 patients (7 lesions) Mean age 69.8 y | Prospective, multicenter, nonrandomized study, 52 patients with metastatic and progressive disease, 9 cutaneous tumors, 5 of which were melanomas (7 applications) | Intravenous bleomycin 15 000IU/cm2 of 1000IU/cm3 followed by EP pulses of 8 to 20min | CR 3 patients, PR 2 patients Side effects were not reported Evidence level 3 |
Abbreviations: CR, complete response; EP, electroporation; OR, objective response; PR, partial response; RCT, randomized clinical trial; SLR, systematic literature review
We found 3 systematic reviews4,9,10 that were analyzed according to the SIGN7 and AMSTAR8 criteria.
The Spratt_2014 review found a high but heterogenous response rate and an improved quality of life. The authors recommended defining treatment algorithms and further studies to establish the specific characteristics of the patients and cutaneous metastases.
All the meta-analyses included in the AETS_2011 review show results clearly in favor of electrochemotherapy versus chemotherapy. The studies took the percentage of patients with complete response (CR) verus other types of response as the outcome measure. The best outcomes were obtained in the late assessment of electrochemotherapy (bleomycin or cisplatin) versus chemotherapy (bleomycin or cisplatin), with a relative risk (RR) of 8.05 (95% CI, 3.43-18.88), as well as in the late assessment (mean follow-up, 9.7 months) of electrochemotherapy (bleomycin) versus chemotherapy (bleomycin), with a RR of 8.48 (95% CI, 3.46-20.79). In the early assessments (mean follow-up, 2.2 months), the RR values were low: 5.01 (95% CI, 2.70-9.32) and 7.56 (95% CI, 2.64-21.60), respectively. In the meta-analysis performed in patients with single diagnosis of melanoma, the outcomes were also in favor of electrochemotherapy versus chemotherapy. In the early assessment of electrochemotherapy (bleomycin or cisplatin) versus chemotherapy (bleomycin or cisplatin), the RR was 4.03 (95% CI, 2.05-7.92) in favor of electrochemotherapy. Likewise, in the later assessment, the RR was 5.95 (95% CI, 1.83-19.33) in favor of electrochemotherapy versus chemotherapy in patients with a single melanoma. With regards the safety of electrochemotherapy, of the 26 studies, 2 did not report the appearance of complications. In the remaining 24 studies, mild complications and side effects were reported.
In a systematic literature review (SLR), Mali_2013 focused on the correlation between tumor size and outcome. The data were obtained from nonrandomized studies. The results show lower effectiveness in tumors larger than 3cm than in smaller tumors (CR and objective response [OR], 59% and 85%, versus 33.3% and 68%, respectively). The authors suggested that the explanation could be the lower drug concentration because of an insufficient application time. The pulses were applied 2min after intratumoral infusion of bleomycin or cisplatin, or within the therapeutic window of 8 to 28min after intravenous bleomycin. Administration of bleomycin to the interstitial space around the tumor attains sufficiently high concentrations, but the plasma concentration declines biexponentially with a mean half-life of distribution of 24 to 30min and a mean clearance of 2-4h.16 The second explanation suggested by the authors was insufficient exposure to the tumor in those larger than 3cm given the irregular vascularization. A third explanation is that a stronger electric field is required.
We identified 4 observational studies with prospective follow-up, with evidence level 3.11–14 Caracò_2013 and Solari_2014 found a significant correlation between response and both number of lesions (fewer than or more than 10) and size (smaller or larger than 2cm), whereas Ricotti_2013 obtained apparently contradictory data, as a higher CR rate was associated with more lesions after the second session.
These are retrospective studies, with a small sample size (n between 5 and 60) and without a comparator group. Solari_2014 provided demographic data on sex and age of the patients. Men tended to be treated more often, perhaps because this is a disease more prevalent among males. All patients were elderly when they received the study treatment.
In Solari_2014 and in Riccoti_2013, criteria for administration were established according to the size of the tumor and the number of nodules to be treated.
They all used a similar protocol: administration of intravenous bleomycin 15 000IU/m2 prior to the electrochemotherapy pulses. Only Skarlatos_2011 also used intratumoral bleomycin in large lesions.
All reported CR rates after treatment of between 10% and 48%, and partial response rates of between 38.3% and 80%. Solari_2014 reported a percentage of patients who did not respond to treatment (15%) or whose diseases progressed (30%). For their part, Caracò_2013 reported a combined response rate for both variables of 13.3%.
None of these authors reported serious side effects; those that were reported were mild and manageable. Follow-up times were short, from 6 to 20 months.
DiscussionThe evidence levels of the studies assessed in this review, 4 cases series and 3 SLRs were medium to low, according to the SIGN classification followed (level 3 for all case series and 1– to 2++ for SLR). The series did not include a control group and were not analyzed in the same time period, the study populations were small, and the studies were not designed to compare effectiveness with other alternatives. The studies were therefore subject to the biases of this type of study. Follow-up was, in general, short, and for this reason, perhaps not all the complications actually caused by the treatment were reported. The internal validity might have been affected by observer bias, as there was no blinding in the reading of the results in any of the studies. No quality-of-life variables were either described or measured, even though these aspects impact health resource use and patient preference. Likewise, we did not find any studies that compared electrochemotherapy with other local or regional therapeutic alternatives. Such a comparison would be of great interest to assess the clinical relevance of the procedure. Nevertheless, alignment of the results for some of the outcome variables analyzed lends consistency to the findings. In fact, the meta-analyses show results in favor of electrochemotherapy compared with chemotherapy, and so these findings could be applicable in Spain.
In this review, we did not find any studies that assessed the organizational or economic impact of the use of electrochemotherapy in clinical practice. Nevertheless, the literature highlights the feasibility, rapid and simple nature of electrochemotherapy, good patient acceptance, and the acceptable cost of treatment. The technique can be considered of great therapeutic potential.
The conclusions of this systematic review are as follows:
- •
Electrochemotherapy could be an effective procedure in local treatment of malignant tumor nodules in terms of objective response, whether complete or partial. Evidence level 3 (low, case series) and 1– to 2++ (medium, SLR).
- •
Electrochemotherapy is easily administered, economical, and well tolerated in that it achieves OR in a high percentage of patients, particularly if there are fewer than 10 lesions and the size is less than 2cm. The procedure can be repeated according to the response of the patient. Evidence level 1–(medium, SLR).
- •
Although the findings show that electrochemotherapy is effective in local control of the tumor lesion, we did not find any evidence that it may influence the natural course of the disease or patient survival, and so it should be considered merely a palliative treatment. Evidence level 3 (low, case series) and 2++ (medium, SLR).
- •
Electrochemotherapy is a safe procedure, without serious adverse effects for the patient. The most frequently reported complications are pain, erythema, muscle contractions, and local edema. Evidence level 1–a 2++ (medium, SLR).
Based on the level of evidence, we can recommend incorporation of electrochemotherapy as palliative therapy for unresectable metastatic melanoma (strength of recommendation: B).
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aguado-Romeo MJ, Benot-López S, Romero-Tabares A. Electroquimioterapia para el tratamiento del melanoma cutáneo locorregionalmente avanzado irresecable. Revisión sistemática. Actas Dermosifiliogr. 2017;108:91–97.