Dark circles (DC), also known as periorbital hyperpigmentation, present as bilateral, semicircular, homogeneous pigmented macules and patches in the periorbital area. They are multifactorial in etiology.1 DC can affect an individual's emotional well-being constituting a common reason for visiting a dermatologist. A variety of treatment options exist.2 However, improvement can be slow and often barely perceptible, posing a therapeutic challenge to the treating dermatologists.
We aimed to evaluate the efficacy and safety of 10% phenol and 20% trichloroacetic acid (TCA) combination peel, a commercial product, in the treatment of DC.
Patients with DC, who were referred from September 2019 to March 2020 to our Dermatology outpatient department, were included in the study. The study protocol was approved by the Ethics Committee of the hospital, and it was carried out in accordance with the Declaration of Helsinki. Pre-peel treatment with 8% glycolic acid once daily was given since 4 weeks before peeling treatment and had been discontinued 2–3 days before the procedure. Patients were asked to maintain their eyes closed during the procedure and the inner canthus of the eye was protected with petroleum jelly, as eye protection is mandatory when chemicals are used around to such a sensitive area. Three layers of croton oil-free 10% phenol and 20% TCA combination peel were applied using cotton tip applicator (allowing a more precise application), to each periorbital area (upper and lower eyelids) for five minutes or less if homogeneous frosting occurred (endpoint of peeling). At that point, they were advised to wash the treated area with pure water. They were subjected to this topical therapy as a single stand-alone treatment. Photographs were taken before the beginning of treatment, as well as after the peeling session and at follow up visits. A Physician's and Patient's global assessment scale was used to evaluate the efficacy of the treatment (0–25% indicated poor response, 25–50% fair, 50–75% good and 75–100% excellent response). All patients were followed up one and three months after their peeling session, evaluating early and delayed adverse events. They were advised to avoid both vigorous eye rubbing and exposure to sunlight. However if exposed the use of both sunscreen and sunglasses was recommended.
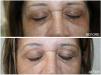
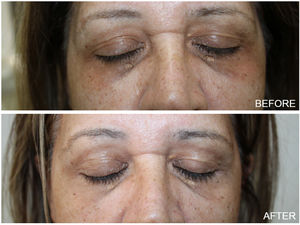
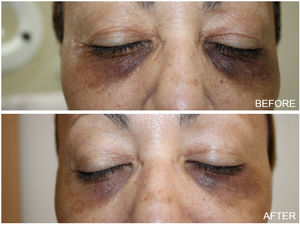
Thirty one female patients with DC were enrolled in the study, aged 43–75 years (mean 47.61±14.81). The majority of them (81%) had Fitzpatrick skin type III and IV. At the end of the study, almost all patients showed aesthetic improvement (Figs. 1 and 2). After examining the photographs of each patient before and after treatment, none of them was classified as ‘worse’ after the treatment. Physicians assessed a poor, fair, good, or excellent improvement in 4%, 25%, 37.2% and 33.8% of patients respectively and Patient Global Assessment rated a poor, fair, good or excellent response in 6.1%, 24%, 44.9% and 25% respectively. Better response was demonstrated by young patients with fair skin phototypes (90% of patients with excellent and good improvement were in their early 40 s while 70% of them had skin type III). The procedure was well-tolerated. With regards to the side effects, no ocular complication (chemical injury to the eye) was noted. Mild discomfort, oedema and transient erythema were quite common during or immediately after the procedure. However these were only temporary. Persistent periorbital oedema was seen in only 5 patients and subsided after a few days (3–5 days post therapy). The exfoliation was most pronounced 7 days after the peel treatment and this scaling persisted up to 14 days.
DC influence patient's quality of life, as they interfere with the face appearance. Chemical peeling has been used alone or in combination with other cosmetic procedures for their treatment.3–5 Among the wide variety of chemical peels available, the ones previously used in treating DC are alpha hydroxy acids, beta hydroxy acids, TCA and ferulic acid.6–8 In the present study, thirty one women with DC were treated with 10% phenol and 20% TCA combination peel and most of them showed a moderate to remarkable improvement. Phenol, also known as carbolic acid, is a deep peeling agent and causes protein denaturation leading to the production of new collagen and elastin.9 Furthermore, it leads to a decrease of melanin granules despite the presence of melanocytes. The classic Baker's formula of phenol includes concentration which varies from 45 to 55 percent leading to a greater risk of adverse events. The croton oil free formulation as well as the low consideration of 10% phenol, used in the present study, makes cardiac monitoring not required while causes frosting and a delayed exfoliation (after 1–2 weeks). TCA in a concentration of 20% acts as a superficial chemical peel and its clinical effect in DC is due to the resultant increase in dermal volume of collagen and melanin reduction through coagulative necrosis of cells.10 The combined use of these agents results in a medium depth peel providing very good cosmetic results in the reduction of pigmentation in DC, alleviating the adverse effects from the usage of each agent alone.
This is the first study using the combination of 10% phenol and 20% TCA in the treatment of DC. According to our results, it seems to be a promising, safe, easy, efficacious, and a cost-effective method treatment of DC.
Conflict of interestsThe authors declare that they have no conflict of interest.