The incidence of anal squamous cell carcinoma has increased alarmingly, particularly in high-risk groups such as men who have sex with men and immunosuppressed patients. Infection with an oncogenic strain of the human papillomavirus in the anal canal or perianal skin leads to anal intraepithelial neoplasias (AIN), progressive dysplastic intraepithelial lesions that are the precursors of anal squamous cell carcinoma. AIN can be diagnosed through cytological screening and biopsy guided by high-resolution anoscopy and can be treated using a range of procedures in an effort to prevent progression to invasive anal carcinoma. Given the recent advances in the understanding of this disease, and the increasing calls from experts for the establishment of screening programs to identify AIN, we review current knowledge on the condition, its diagnosis, and treatment from the point of view of dermatology.
La incidencia de carcinoma epidermoide anal ha aumentado de forma alarmante, especialmente en grupos de riesgo como son los pacientes homosexuales y los inmunosuprimidos. La infección por un genotipo oncogénico del virus del papiloma humano (VPH) en el canal anal o en la piel perianal, desencadena una progresión de lesiones displásicas intraepiteliales (neoplasia intraepitelial anal o NIA), que son las precursoras del carcinoma epidermoide anal.
La NIA puede diagnosticarse a través del cribado mediante citología y biopsia guiada por anoscopia de alta resolución, y puede tratarse mediante diferentes procedimientos, con el fin de evitar la progresión a carcinoma anal invasivo.
En vista de los recientes avances en el conocimiento de esta patología, y de que cada vez son más los expertos que recomiendan la puesta en marcha de programas de cribado de la NIA, revisamos el concepto actual de la misma, su diagnóstico y tratamiento desde una perspectiva dermatológica.
The incidence of anal squamous cell carcinoma (SCC) has increased considerably in the past decade, mainly due to the growing number of cases in high-risk groups such as men who have sex with men (MSM), immunosuppressed individuals, and women with a history of cervical dysplasia.
As is the case with cervical cancer, anal SCC arises from intraepithelial dysplastic lesions (anal intraepithelial neoplasia, or AIN) at the squamocolumnar junction of the epithelium of the anal canal. These dysplastic lesions are caused by infection by the oncogenic human papillomavirus (HPV) and by HPV-16 in particular.
AIN can be diagnosed by cytologic screening and high-resolution anoscopy, and the many therapeutic advances in this field have now provided numerous options for preventing progression to invasive anal SCC.
Although the utility of screening for AIN and treating it in high-risk patients is still a subject of debate, screening and treatment programs are becoming increasingly common and will probably become widespread in dermatology practices in the future.
Epidemiology of Anal SCCAnal SCC is an uncommon cancer that accounts for less than 5% of all gastrointestinal neoplasms. Its incidence in healthy males in the United States is 0.8 cases per 100 000 population.1 Unfortunately there are no reliable data on the incidence or prevalence of anal SCC in Spain as there are no official registers. Nonetheless, recent epidemiological studies conducted outside Spain (mainly in Europe2–4 and the United States5) have revealed that the incidence of anal SCC is rising, with estimates indicating a 2% annual increase in the past 10 years; the increase in high-risk groups has been particularly dramatic.
The incidence of anal SCC varies in different population subgroups. In MSM, for example, an estimated 35 new cases per 100 000 individuals are found each year.6,7 Although this figure is half that reported for patients infected with human immunodeficiency virus (HIV), it is similar to the incidence of cervical cancer in women before the introduction of widespread cytologic screening.
More alarming, however, is the situation for MSM with HIV, who have now become the group most at risk of developing this disease; the incidence of anal SCC in this population is estimated at 70 to 128 cases per 100 000 person-years.8–10 Several studies have analyzed changes in the incidence of anal SCC in relation to the AIDS epidemic of 1980 to 1990 and the introduction of highly active antiretroviral therapy (HAART) in 1996. Practically all these studies concluded that HAART has not reduced the incidence of anal SCC as it reduced other AIDS-related tumors such as Kaposi sarcoma, non-Hodgkin lymphoma, and cytomegalovirus retinitis6; in fact, the incidence of anal SCC has increased markedly in the post-HAART era.8,10,11
Because HAART appears to have no effect on the natural history of anal SCC and considerably increases survival, one would expect the incidence of anal SCC to continue to rise. Anal SCC is now the most common non-AIDS-defining cancer12 and has become one of the main health problems of HIV-positive individuals.Other groups at risk of anal SCC are individuals with immunocompromised systems for reasons other than HIV infection, such as those who have received immunosuppressive treatment following a solid-organ transplant. Several studies have reported that patients who have undergone kidney transplantation, for example, are 10 times more likely than the general population to develop anal SCC.12–14
The risk of anal SCC in women is associated with the presence of other tumors in the anogenital region; this risk probably stems from the close proximity of the cervix and anal canal, which both act as viral reservoirs, favoring mutual reinfection regardless of which site was infected first. Patients who have already had neoplasia in the anogenital region will thus be at considerably higher risk than the general population. One recent study reported that 12.2% of patients with a history of cervical, vulvar, or vaginal intraepithelial neoplasia who underwent anal cytology and high-resolution anoscopy had AIN.15 Obviously this risk will be much higher in immunosuppressed individuals, and higher still if they are HIV-infected.
EtiopathogenyLong-term studies have yet to establish a definitive model that provides a compelling explanation of the natural history of anal neoplasia. By analogy with cervical cancer, and based on the results of recent studies,9,16–18 it is currently accepted that intraepithelial dysplastic lesions are precursors of invasive tumor lesions.
Evidence suggests that HPV plays a key role in the etiopathogenesis of up to 93% of anal carcinomas. Patients with anal SCC and its precursor, high-grade AIN, have been found to have a significantly higher prevalence of HPV-16 and HPV-18 infection.19–21 In the cervix, the virus infects the epithelium of the squamocolumnar junction (the cervical transformation zone), but in the anal canal it tends to infect the transformation zone between the columnar epithelium of the rectum and the stratified epithelium of the anoderm, which is precisely where the dysplastic changes that characterize AIN occur.
It is therefore easy to understand why AIN and anal SCC are so common in HIV-positive MSM. On the one hand, a prevalence of HPV infection as high as 60% is well documented in MSM and rises to 93% in MSM with HIV infection22–25; these higher rates are partly explained by longer survival and hence longer HPV exposure.26
On the other hand, AIN is also closely associated with different degrees of compromised immunity, particularly in relation to HIV infection. The interaction between HIV and HPV has been said to be related to various possible mechanisms, including attenuation of cellular response to HPV antigens in HIV-positive patients, aberrant expression of cytokines (interleukin 6) that regulate the expression of HPV genes, increased expression of local growth factors, and a direct effect of HIV on the expression of HPV oncogenes E6 and E7.25
The Natural History of AINAIN as the precursor of anal SCC was first described by Fenger and Nielsen27 in 1981.
Anal biopsy is the gold standard for a diagnosis of AIN, which is based on detection of dysplasia, classified according to the depth of epithelial involvement. A grade classification of 1 to 3 is assigned based on how many layers of the epithelium have dysplastic changes.
AIN can also be detected by anal cytology, just as dysplastic lesions of the uterine cervix can be detected by cervical cytology. Accordingly, the Bethesda28 criteria can be used to distinguish between low-grade and high-grade lesions. Cytologic findings, however, should always be confirmed histologically via anal biopsy, which always provides the definitive diagnosis.
While fewer studies have analyzed the natural history of AIN than are available for cervical dysplasia, there is no doubt that high-grade AIN (grades 2 and 3) is a precursor of anal SCC. Various authors have documented the progression of grade 3 dysplasia to carcinoma in situ, using long-term histologic follow-up studies.9,16–18 Despite these observations, however, and despite evidence of progression of high-grade AIN to anal carcinoma, more studies are required to clarify the clinical significance of these dysplastic lesions.
Untreated low-grade (grade 1) AIN can regress spontaneously or progress to higher-grade lesions.29 Low-grade AIN may progress to high-grade AIN, or even to anal SCC in approximately 10% of patients.26 Scholefield et al18 reported that 15.6% of patients (5/32) with grade 3 AIN developed invasive anal SCC over a period of 18 months.
Logically, those with the highest risk of AIN also have the highest risk of anal SCC. The estimated prevalence of AIN in MSM, for example, is 35%30 (72% to 81% in HIV-positive individuals17,23,24,30 and 20% in patients with grade 3 cervical intraepithelial lesions or cervical cancer); AIN is also more common in patients with immunosuppression for reasons other than HIV infection (eg, patients who have undergone kidney transplantation).12,15,19 Furthermore, approximately 10% of patients with anal condyloma have AIN of varying grades, demonstrating why histologic examination is important in the assessment of anal and perianal condyloma.19,31
The increased risk of AIN in HIV-positive individuals was highlighted in 2 recent studies by the same group, who showed that 81% of HIV-positive MSM had AIN (grades 1, 2, or 3) and that 52% had high-grade AIN.23,24 A study performed in Germany in 446 HIV-infected MSM found that 72% had AIN of some degree of severity and 35% had high-grade AIN.16
An association between CD4 expression and the risk of AIN has been shown in HIV-positive individuals, in whom risk rises as CD4 cell counts fall.24,32 Duration of HIV infection (ie, of impaired immunity) has also been proposed as a possible risk factor for anal SCC.33 A recent study in 4901 HIV-positive patients reported a 12-fold higher prevalence of anal SCC in those who had been infected for over 15 years compared to those who had been infected for less than 5 years (P<.01).10 Observations such as these are very interesting in terms of helping to identify appropriate candidates for screening (ie, individuals with a higher risk of developing anal carcinoma). Based on the above observation, the target population would be MSM with a longer duration of HIV infection.
The natural course of AIN (progression or regression) appears to be influenced by HIV infection, the oncogenic potential of HPV infection, HPV viral load, multiple HPV infection, and the host's immune status.34
Several studies have shown that disease progresses more rapidly in HIV-positive patients, who have lower rates of spontaneous regression and more frequent progression to more advanced stages of disease.24,35 Specifically, these studies have shown that 32% of HIV-positive MSM with normal cytology and 52% of those with low-grade AIN develop high-grade lesions in just 4 years.
The incidence of anal SCC in immunocompetent MSM, while still high, is lower than would be expected considering the extremely high prevalence of AIN in these individuals36 a pattern that stands in contrasts with that of HIV-infected MSM. The probable explanation is that spontaneous regression of AIN is much more common in immunocompetent individuals.
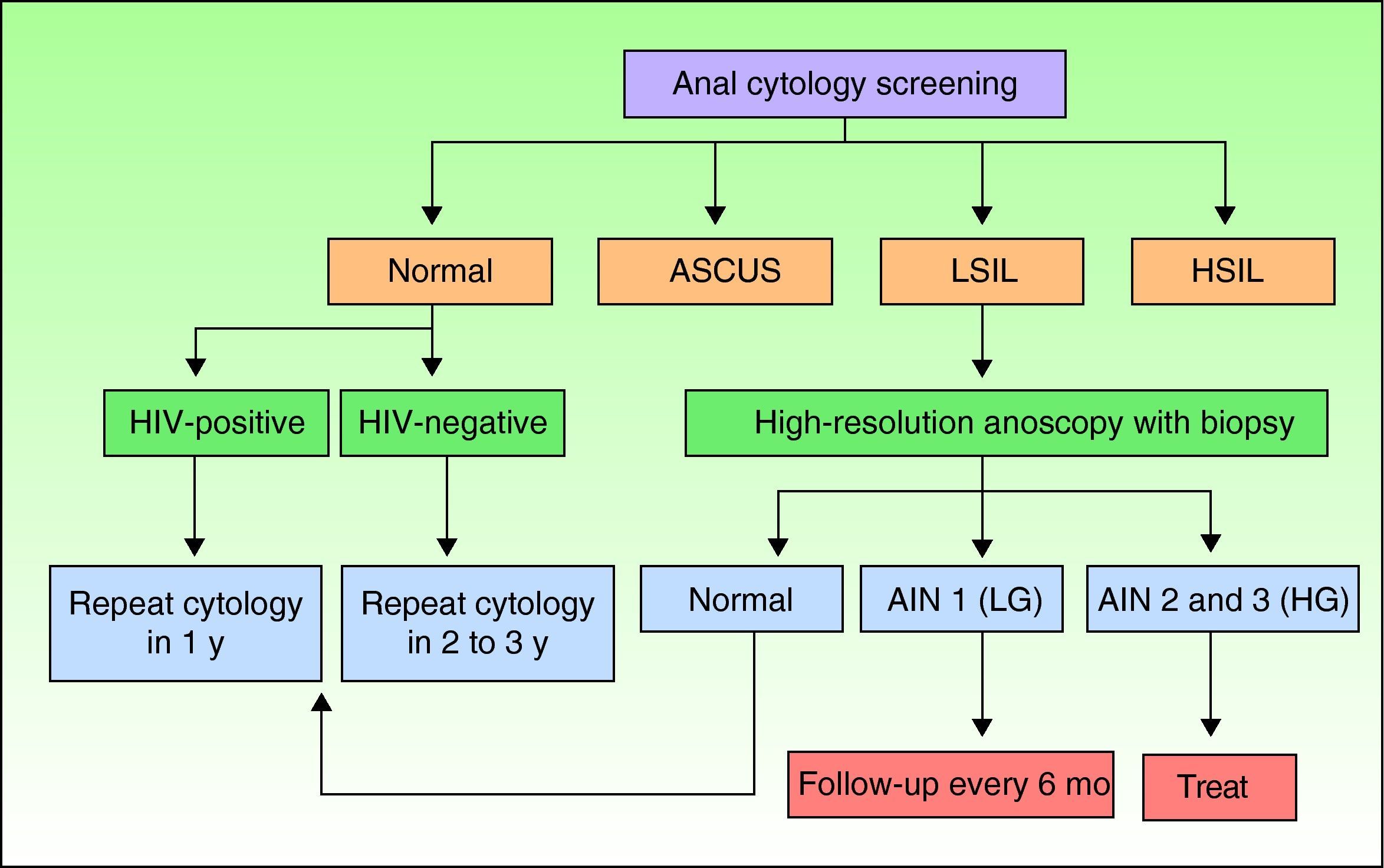
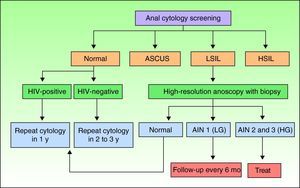
Screening for AIN by Cytology and High-Resolution AnoscopyAnal cytology is the first screening test that should be performed in individuals at risk of AIN, and those with abnormal findings should be referred for high-resolution anoscopy-guided biopsy (Fig. 1).
Screening algorithm adapted from Chin-Hong and Palefsky.9 AIN indicates anal intraepithelial neoplasia; ASCUS, atypical squamous cells of undetermined significance; HG, high-grade; HIV, human immunodeficiency virus; HSIL, high-grade squamous intraepithelial lesions; LG, low-grade; LSIL, low-grade squamous intraepithelial lesions.
Anal cytology is a simple, minimally invasive procedure used to collect cells for cytopathologic examination. When collected in a liquid medium, the sample can also be used for quantitative HPV testing.
The procedure involves introducing a cytobrush into the anal canal to a distance of 3cm and rotating it to increase contact with the canal walls. The brush is then submerged in a vial containing a preservative solution (eg, PreservCyt) to release the cells for analysis. Part of the sample is processed for HPV DNA testing by hybrid capture.
Anal cytology has improved considerably in recent years and now offers sensitivity and specificity rates that are at least comparable to those of cervical cytology. When a liquid medium is used, a sensitivity of 69% to 92% is possible, with a specificity of 32% to 59%.36–40 Nonetheless, like cervical cytology, it does not adequately predict the degree of histologic involvement. High-grade dysplasia detected by anal cytology has a high predictive value for histologically confirmed high-grade dysplasia. Low-grade cytologic abnormalities, in contrast, are not a reliable reflection of the true extent of disease. Atypical squamous cells of undetermined significance (ASCUS) and low-grade dysplasia, for example, may be detected in histologically confirmed high-grade dysplasia. Any abnormal cytology result, therefore, should be followed up with high-resolution anoscopy and biopsy of suspicious areas to confirm AIN and determine the level of dysplasia.
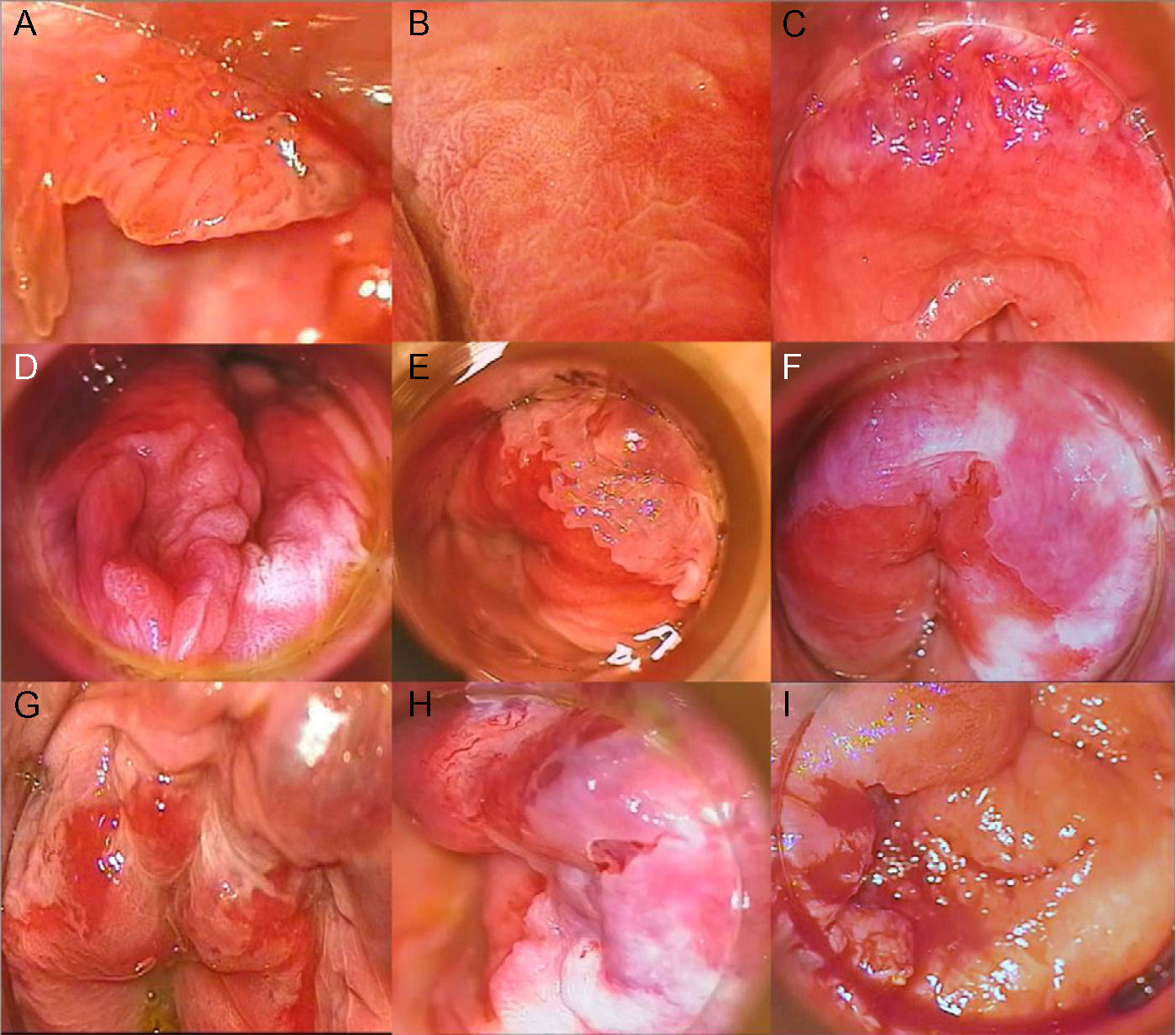
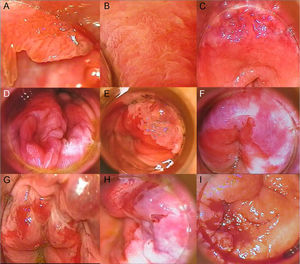
High-Resolution Anoscopy-Guided BiopsyHigh-resolution anoscopy-guided biopsy is the gold standard for diagnosing AIN. In this procedure, the transformation zone of the anal canal is examined through a colposcope, which lights and magnifies the surface. A gauze pad soaked in 3% acetic acid is inserted into the canal, where it is left in contact with the mucosa for several minutes. Intraepithelial lesions tend to acquire a whitish color when they come in contact with this solution, thus helping to identify suspicious areas for biopsy. Subsequent staining with Lugol solution can also be performed. AIN should be suspected on detection of acetowhitening, plaques that do not take up Lugol solution, or areas of the mucosa with abnormal vascular patterns. Figure 2 shows grades 1, 2, and 3 AIN as seen by high-resolution anoscopy (Fig. 2A-I). Histologic evaluation of samples obtained by punch biopsy is the method of choice for diagnosing and grading AIN.22
A, Condyloma in a patient with grade 1 anal intraepithelial neoplasia (AIN). B, Grade 2 AIN showing a papillomatous pattern with vascular punctation. C, Acetowhite plaque with abnormal vascularization in the upper right and left quadrants in a patient diagnosed with grade 2 AIN. D, Extensive acetowhite plaque with a cobblestone appearance in lower right and left quadrants in a patient with grade 2 AIN. E, Circumscribed acetowhite plaque in the upper left quadrant, corresponding to grade 2 AIN. F, Circumferential acetowhite plaques in a patient with grade 3 AIN. G, Acetowhite plaques and friable mucosa with hemorrhagic, erosive areas in a patient with grade 3 AIN. H, Acetowhite plaque and thick, tortuous vessels in upper quadrants. I, Exudative, hemorrhagic mass in a patient with infiltrating squamous cell carcinoma.
Opinions are divided on the diagnostic value of HPV testing in AIN screening because of the extremely high prevalence of HPV in the highest risk groups such as HIV-positive MSM. It is widely known that most HIV-positive MSM have multiple HPV infections, and infection rates vary very little between those who have AIN and those who do not.
HPV testing is used to determine the need for high-resolution anoscopy in women with a cervical cytologic diagnosis of ASCUS. In HIV-positive MSM with anal ASCUS, HPV testing is probably not useful as it does not increase the specificity for detecting AIN39 as most of these patients will harbor an oncogenic HPV variant. It has been suggested, however, that it might be a useful addition in HIV-negative MSM, in whom the sensitivity of anal cytology is lower.40 HPV testing in other risk groups such as women with a history of cervical dysplasia might also be useful due to the lower prevalence of anal HPV infection in this group.
The inclusion of HPV testing as a routine part of AIN screening might also be of value because of its negative predictive value. In other words, because dysplasia is not found in patients who do not have oncogenic HPV infection,39 a negative result would indicate that high-resolution anoscopy could be avoided. Unfortunately, this occurs only in a very small percentage of at-risk patients.
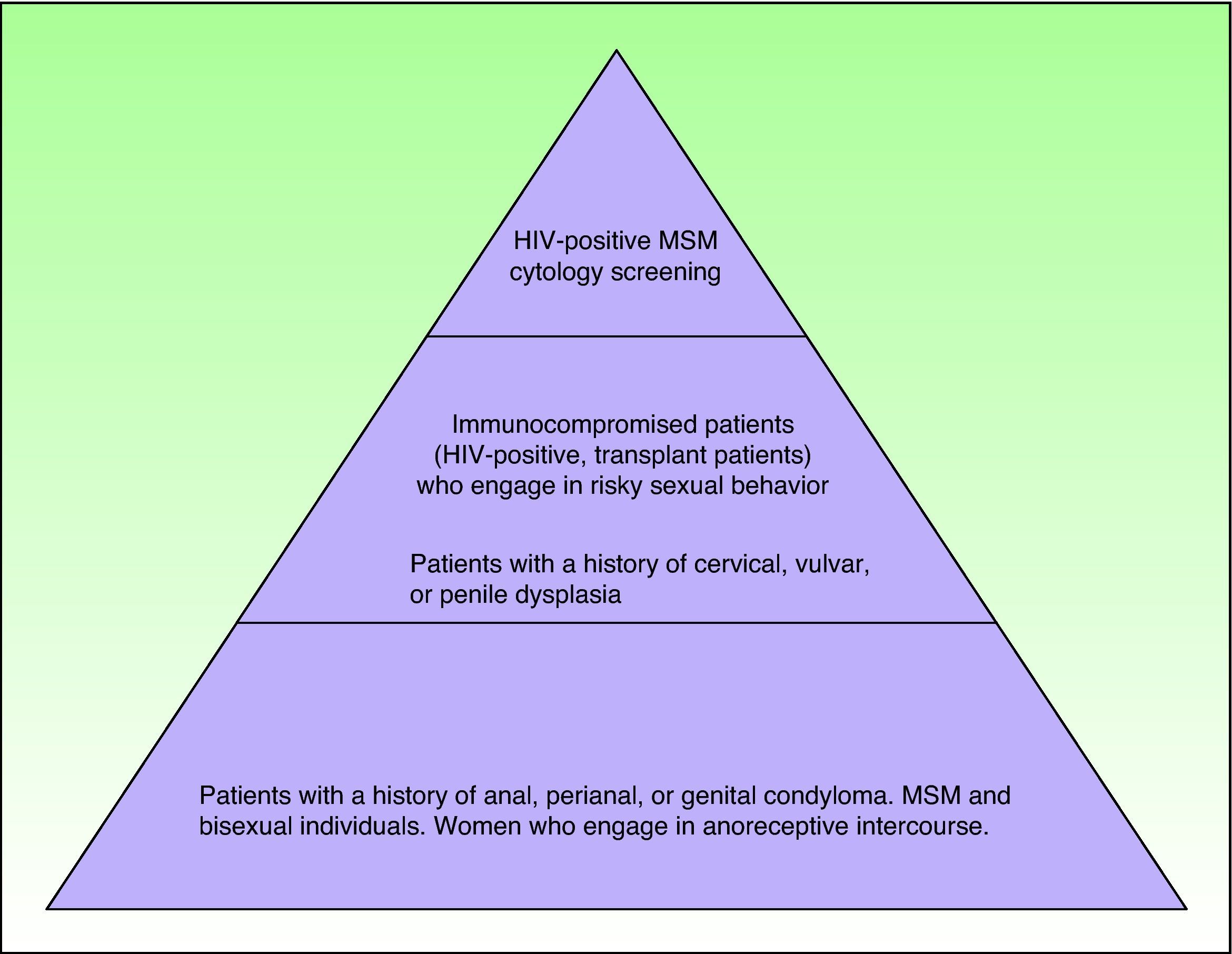
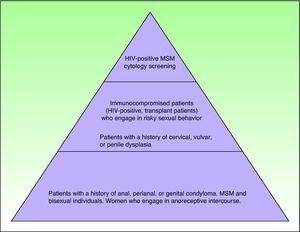
Which At-Risk Patients Should Be Considered for Screening?As mentioned previously, a number of major groups have an increased risk of developing AIN in association with HIV infection. Exactly which patients should be referred for screening, however, is a complicated decision in which cost-effectiveness is an important consideration. Figure 3 shows which individuals are at risk, although it should be noted that studies to date have only shown screening to be cost-effective when performed annually in HIV-positive patients and every 2 to 3 years in HIV-negative MSM.41,42
Several official HIV guidelines such as that published by the New York State Department of Health AIDS Institute43 recommend systematically screening for AIN in patients with HIV infection. Guidelines from the Centers for Disease Control and Prevention simply state that cytology is used by many experts to screen for AIN in HIV-positive patients but do not include any specific recommendations in this respect.44
Screening AlgorithmsThere are no universal screening algorithms for AIN and most authors use the protocol proposed by Chin-Hong and Palefsky9 (Fig. 1). According to this protocol, at-risk individuals should undergo anal cytology screening following the procedure described previously. In the event of a negative result, the test should be repeated after 1 year in HIV-positive individuals and after 2 to 3 years in HIV-negative individuals. High-resolution anoscopy-guided biopsy is indicated in patients with abnormal anal cytology. If the lesions are grade 1, the anoscopy should be repeated in 6 months, but if they are grade 2 or 3, treatment is recommended. Individuals with low-grade cytology but normal anoscopy findings should be scheduled for repeat cytology in 6 months. In the rare situation where a patient has high-grade cytology but a normal anoscopy, blind biopsy should be performed and cytologic examination repeated shortly afterwards.
Limitations and Future Prospects of AIN ScreeningAs mentioned previously, anal cytology has similar sensitivity and specificity to cervical cytology, although higher rates have been reported in patients with disease in more than 1 quadrant of the anal mucosa and in HIV-positive patients, particularly when their CD4 cell count is low.45
Anal cytology, like cervical cytology, has its limitations for screening, however, because it does not detect all high-grade AIN. Furthermore, many healthy patients are unnecessarily referred for high-resolution anoscopy.
Because of these limitations, molecular diagnostic techniques are being investigated in an effort to improve the sensitivity and specificity of AIN screening. Recent proposals include the use of cytologic, histologic, and microbiologic markers to help identify candidates for high-resolution anoscopy. Of note are proteins that can be used as surrogate markers of cell cycle deregulation, biomarkers of DNA damage, gamma tubulin and beta defensin levels, HPV typing, and the identification of integrated forms of HPV.46,47
Finally, in view of the high incidence of AIN in certain risk groups, some authors have even proposed that all high-risk individuals should go directly to high-resolution anoscopy screening.39
Treating AINAIN represents a therapeutic challenge for several reasons. Firstly, all current treatments are associated with a risk of recurrence and metachronous lesions. Treatment, unfortunately, does not cure the viral infection, meaning that patients need to be monitored for long periods of time because of the risks associated with persistent infection by oncogenic forms of HPV.
Secondly, there is no treatment of choice for AIN. Approaches vary according to the location and extent of disease and the availability of resources and local expertise.48
It is not considered necessary to treat low-grade AIN unless the patient has symptoms or feels particularly anxious about the disease. High-grade AIN, in contrast, should be treated to prevent progression to invasive carcinoma, except in cases in which treatment might result in more complications than the disease itself. In such cases, 3-monthly to 6-monthly follow-up with high-resolution anoscopy and digital rectal examination is recommended to ensure timely detection of progression to invasive carcinoma.
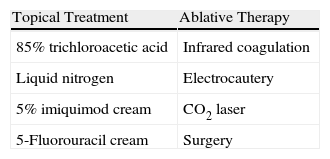
AIN can be treated with topical agents, ablative therapy, or surgery48 (Table 1).
The treatment of local AIN is relatively simple and there are many options, including cryotherapy, trichloroacetic acid, infrared coagulation, electrocautery, CO2 laser, and surgery.48
Treatment is more complicated in the case of more extensive disease. Options include patient-administered topical treatments with curative intent or to reduce the number of lesions for subsequent treatment with ablative techniques such as infrared coagulation. The main topical treatments are imiquimod49–52 and 5-fluorouracil53 creams, which are associated with histologic regression rates of 74% and 57%, respectively.49–53 Both treatments reduce the viral load of oncogenic HPV genotypes but are also associated with high recurrence rates: 58% in the case of imiquimod (30 months post-treatment)50 and 50% in the case of 5-fluorouracil (6 months post-treatment).53 Most recurrences, however, are detected at sites other the initial AIN site and are associated with high-risk HPV genotypes other than those detected in the initial examination. In other words, most recurrences are due to HPV reinfection.
One particularly interesting recent study was a double-blind, placebo-controlled, randomized trial of imiquimod which included the option of a second 4-month treatment cycle for nonresponders.52 During this second phase, which lasted at least 36 months, 61% of patients achieved complete remission.
Infrared coagulation, which involves the delivery of heat to induce coagulation, also holds much promise as a treatment for AIN.54–56 This modality can be used to treat extensive disease in office-based settings (with or without anesthesia depending on the site of the lesions), and unlike treatment with CO2 lasers, does not require the use of an extractor as it does not produce smoke. Several retrospective studies have indicated that infrared coagulation is a safe procedure that achieves response rates of 60% to 70% when used on specific AIN lesions.55 Higher response rates have been reported in HIV-negative patients and individuals who undergo several treatment sessions.55
The Role of HPV VaccinesConsidering that over 70% of SCCs are caused by HVP-16, vaccination is a promising primary preventive measure against AIN and anal SCC. In October 2009, the US Food and Drug Administration approved a quadrivalent HPV vaccine for the prevention of condylomas caused by HPV types 6, 11, 16, and 18 in males aged between 9 and 26 years. One of the main problems associated with the use of this vaccine to prevent anal carcinoma in high-risk groups of men is that it is most effective in men who have not yet been infected by HPV. In other words, it should ideally be administered prior to sexual debut.
Because it is impossible to identify at-risk individuals before they become sexually active, it would be necessary, at least hypothetically, to vaccinate all preadolescent males in order to reduce the incidence of anal carcinoma.48 For this to be viable, however, it would first be necessary to determine whether universal vaccination would be cost-effective, particularly given the high cost of the vaccine and the relatively low incidence of anal SCC.
According to the results of a recent study by Kimm57 designed to estimate the cost-effectiveness of the quadrivalent HPV vaccine administered at the ages of 12 years, 20 years, or 26 years in terms of preventing AIN and anal SCC in MSM, the cost-benefit ratio was highest for vaccination at 12 years, but the intervention was also cost-effective at 20 and 26 years, ie, at ages when individuals would be more likely to ask for the vaccination.
The fact that multiple HPV infection—like infection by other sexually transmitted diseases—has been found to be independently associated with HIV acquisition in MSM58 would be another argument in favor of vaccinating young males.
ConclusionsThe value of targeted and universal AIN screening has generated much debate in the past 10 years and none of the relevant national or international guidelines recommend routine AIN screening. Nevertheless, the results of population-based studies and the experience of dermatologists in the past decade bear witness to the fact that anal carcinoma is increasing at an alarming rate, particularly in HIV-positive patients.
We must offer patients the best preventive treatment possible based on the knowledge accumulated to date on the role of HPV in anal carcinoma and the natural history of AIN. Anal cytology is a simple, noninvasive technique, but before extending its use to all at-risk populations, we must first be able to provide a definitive diagnosis and offer treatment in the event of cytologic abnormalities. In other words, patients at centers that do not offer high-resolution anoscopy should be referred to centers that do. When this is not possible, high-risk patients should be followed closely and tests should include digital rectal examination to test for masses suggestive of anal carcinoma.
In our opinion, and in line with practices in other European countries, AIN screening can and should be offered by dermatologists, who are, indeed, specialists in sexually transmitted diseases, with the aim of diagnosing and treating intraepithelial lesions associated with HPV. That said, the approach should be multidisciplinary and involve close collaboration with specialists from other departments such as internal medicine, general surgery, digestive surgery, microbiology, and pathology to offer patients the best possible care.
Finally, further studies are needed to develop an optimal AIN screening protocol and, more importantly, to establish appropriate guidelines and treatment.
Please cite this article as: Sendagorta E, et al. Detección precoz de la neoplasia intraepitelial anal en pacientes de alto riesgo. Actas Dermosifiliogr.2011;102:757-765.