Imiquimod is an immunomodulating agent available in 3.75% or 5% cream. It has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of viral warts (VW), actinic keratosis (AK) and superficial basal cell carcinomas (sBCC): however, it is widely used off-label for other infective and neoplastic diseases.1,2 Its mechanism of action implies the activation of innate and acquired immune responses by increasing relapse of interferon-α (IFN-α), tumor necrosis factor-α (TNF-α) and cytokines like interleukin-1 (IL-1) and IL-17.1 We already know that this activation of the immune system and increased activity of T-helper 1 cells can induce some cutaneous immune-related adverse events such as psoriasis and non-cutaneous adverse events such as fever, asthenia, myalgia, arthralgia and headache.1–3
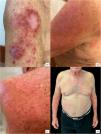
A 65-year-old man presented with a sBCC on the right arm. Treatment with imiquimod 5% cream was initiated. After 5 weeks of once-daily application, he developed an erythematous, vesicular, and pruritic eruption on the chest, back, and arms, clinically consistent with acute eczema. Blood tests showed high levels of total IgE (1868U/mL). We performed an incisional biopsy, and the histological examination confirmed our hypothesis. The patient reported a past medical history of atopy during childhood, with allergic rhino-conjunctivitis, asthma and atopic dermatitis, all improved during adolescence. The patient underwent patch testing with the standard SIDAPA series supplemented with imiquimod excipients (isostearic acid, benzyl alcohol, cetyl alcohol, stearyl alcohol, white Vaseline, polysorbate 60, sorbitan stearate, glycerol, methyl hydroxybenzoate, propyl hydroxybenzoate and xanthan gum). Every substance tested came out negative. Imiquimod application was discontinued, followed by the administration of oral desloratadine, topical mometasone furoate and a short course of systemic methylprednisolone, all associated with emollient cream. After 2 weeks the patient showed clinical remission of dermatitis, followed by discontinuation of the therapy. During the following months, he reported several relapses managed with topical mometasone furoate. At the 12-month follow up after interrupting treatment with imiquimod, moderate dermatitis still remains along with cutaneous xerosis and itch. We repeated the evaluation of total serum IgE, that revealed a very high value (3069U/mL) (Fig. 1).
Since there is a clear temporal association between the initiation of imiquimod and the development of diffuse eczema, we interpret this skin reaction as an adverse event to this drug. This type of cutaneous adverse event after application of imiquimod is already described in literature but, to our knowledge, only 1 case has been reported.4 The most common adverse events are erythema, scabbing, itching, pain, tenderness, erosion and ulceration on the site of application, fever and flu-like symptoms,3 all manageable without therapy discontinuation which occurs in only 2–3% of the cases.2 All these reactions are time and dose dependent but we do not know if severity is correlated with a better clinical response.2 Less common are autoimmune disorders such as pemphigus foliaceus, autoimmune spondyloarthropathy and vitiligo, angioedema, and erythema multiforme.2 Numerous cases of psoriasis developing during treatment with the same drug have been reported,5 a finding that may be explained by imiquimod-induced enhancement of T-helper 1 (Th1) activity, a pathway implicated in psoriasis.5 Imiquimod is also known to stimulate interferon-α (IFN-α) production, and in atopic dermatitis the immune response is predominantly driven by T-helper 2 (Th2) cytokines. The shift toward a Th1-skewed profile may help explain the development of widespread eczematous reactions in predisposed individuals.4 So we hypothesize that the mechanism of action of imiquimod could increase the risk of developing eczematous lesions in atopic patients. Therefore, we recommend asking every patient if they have a past medical history of allergies or atopy before starting this kind of drug. If so, we suggest performing some screening exams, such as total and specific IgE panels and highlight the need for caution when using imiquimod 5% cream in such patients.
Furthermore, additional studies are needed to clarify the pathophysiologic mechanisms underlying the association between imiquimod and these types of adverse reactions.
Conflict of interestThe authors declare no conflict of interest.