Ringworm is highly prevalent in our setting and is frequently observed in our routine clinical practice. Diagnostic confirmation depends on techniques that are not always accessible (PCR), with highly variable sensitivity depending on the observer (direct microscopy) or delayed results (culture, histopathology). Recently, an immunochromatography-based rapid test (Diafactory®) for the antigenic detection of dermatophytes has been developed. This diagnostic tool can help diagnose ringworm, allowing early initiation of treatment and fewer consultation visits.
ObjectiveTo determine the sensitivity and specificity of the rapid antigen detection test compared to conventional culture.
Material and methodsFor a full year, 333 nail samples were collected from patients with suspected onychomycosis. The rapid test and the conventional culture were simultaneously performed on each sample. Those with a positive antigenic test result began treatment early. The remaining patients had appointments for serial cultures and subsequent medical consultation to evaluate the results.
ResultsCompared to conventional culture, the sensitivity and specificity rates of the rapid antigen detection test are 97.2% and 80.7%, respectively.
ConclusionThe effectiveness of the rapid antigen detection test is similar to that of conventional culture for the detection of dermatophytes in nail samples. It is a quick and simple diagnostic technique that reduces the number of patient visits to the hospital, and allows early treatment start.
La tiña ungueal es muy prevalente en nuestro medio y se observa con frecuencia en la práctica diaria. La confirmación diagnóstica depende de técnicas no siempre accesibles (PCR), con sensibilidad muy variable en función del experto (microscopia directa) o que ofrecen un resultado tardío (cultivo, histopatología). Recientemente se ha desarrollado un test rápido de detección antigénica de dermatofitos, basado en la inmunocromatografía, denominado Diafactory®. Esta herramienta diagnóstica podría ser útil en el diagnóstico de la tiña ungueal, permitiendo el inicio precoz del tratamiento y una disminución de las visitas a consulta.
ObjetivoDeterminar la sensibilidad y la especificidad del test rápido de detección antigénica en comparación con el cultivo.
Material y métodoDurante un periodo de un año, se recogen 333 muestras ungueales de pacientes con sospecha de onicomicosis. Se realiza de forma simultánea el test rápido y el cultivo en cada muestra. Aquellos con resultado en test antigénico positivo inician el tratamiento de forma precoz. El resto de pacientes reciben citas para la realización de cultivos seriados y posterior consulta médica para la valoración de los resultados.
ResultadosLa sensibilidad y especificidad del test rápido de detección antigénica en comparación con el cultivo es del 97,2% y 80,7%, respectivamente.
ConclusiónLa efectividad del test rápido de detección antigénica es comparable con la del cultivo para la detección de dermatofitos en muestras ungueales. Es una técnica diagnóstica rápida y sencilla que permite una reducción en el número de visitas de los pacientes al hospital, así como un inicio precoz del tratamiento.
Onychomycosis is a superficial fungal infection with a high prevalence, affecting between 10% and 40% of the European population, and its incidence increases with age.1 Toenails are more frequently affected than fingernails. The most common causal pathogen is dermatophytes, with Trichophyton rubrum being the most widespread. Dermatophytes are responsible for 89% up to 90% of onychomycosis cases and cause onychomycosis.2
From a clinical perspective, onychomycosis manifests with thickening of the nail plate, hyperkeratosis, discoloration, distal onycholysis, and splinter hemorrhages. The most common onychoscopic patterns observed in onychomycosis are distal onycholysis with an irregular proximal advancing edge, chromonychia, and increased longitudinal striations.3 Nail dermoscopy is an additional, simple, rapid, and cost-effective diagnostic tool that can increase diagnostic accuracy.4 However, there is no pathognomonic sign to confirm the clinical diagnosis. Differential diagnoses include traumatic onychopathy, nail psoriasis, nail lichen planus, and other inflammatory skin diseases.5
Treatment consists of oral systemic antifungals such as terbinafine, fluconazole, or itraconazole. These are prolonged therapies that go on for, at least, 12 weeks.6 The most significant adverse effects of these drugs are hepatotoxicity and cytopenias. Therefore, diagnostic confirmation is necessary before starting therapy to avoid overtreatment due to diagnostic errors.7
The diagnosis of this common disease is established using techniques such as culture, direct observation or fresh examination, histopathology, or polymerase chain reaction (PCR). Although the combination of culture and fresh examination has been the gold standard in recent years, its false-negative rate ranges from 15% up to 80%.8 Currently, there is no consensus on the most appropriate combination of diagnostic tests to increase sensitivity and specificity.9
Both culture and fresh examination have the advantage of being available in most health care centers. The disadvantage of culture is that it is a slow diagnostic test, offering results in 3 to 4 weeks and requiring repeated testing to achieve acceptable sensitivity. On the other hand, fresh examination has highly variable sensitivity, depending on the observer's experience with the microscope. Histological examination, while very reliable, is an invasive and complex method. PCR has excellent sensitivity and specificity but is a costly technique and is not available in most centers.10
At Hospital de Fuenlabrada, Madrid, Spain we base diagnostic confirmation of onychomycosis on 3 serial cultures, which requires the patient to return 4 weeks after taking the last sample for culture. We review the results and prescribe the necessary therapy, delaying its start.
Therefore, it seems necessary to find a fast, simple, minimally invasive, and cost-effective method to diagnose and start treatment as soon as possible, avoiding unnecessary medical appointments and making better use of available resources.
The rapid antigen detection test, Diafactory®, is a diagnostic technique developed in Japan and recently introduced in Europe, arriving in Spain in 2018. Diafactory® is based on immunochromatography and uses antibodies that target the epitope present in 7 species of dermatophytes (Trichophyton, Microsporum, and Epidermophyton). Additionally, it has the advantage of providing a result that is not affected by the most widely used antifungal agents—terbinafine, itraconazole, griseofulvin—so prior treatment with any of these drugs does not interfere with the test result.11,12
Endpoints- 1.
Evaluate the sensitivity (S) and specificity (E) of the rapid antigen detection test, Diafactory®, as a diagnostic technique for onychomycosis, considering fungal culture as the reference diagnostic technique.
- ∘
Sensitivity is the ratio between the number of positive antigen tests and the number of positive cultures where a species of dermatophyte has grown.
- ∘
Specificity is the ratio between the number of negative antigen tests and the number of cultures where a non-dermatophyte fungus has grown.
- 2.
Evaluate the negative predictive value (NPV) and the positive predictive value (PPV) of the rapid antigen detection test, Diafactory®, as a diagnostic technique for onychomycosis, considering fungal culture as the reference diagnostic technique.
A total of 333 nail samples were collected from patients with nail disorders and suspected onychomycosis as part of the differential diagnosis at the Dermatology Department of Hospital Universitario de Fuenlabrada over the course of 1 year (from January 2021 through January 2022).
Samples were collected by trained nursing staff from the dermatology department. The method of sample collection depended on the specific type of nail involvement. If onychomycosis was distal and lateral subungual, the area of onycholysis or the tip was removed to take a deep sample from beneath the nail, as close as possible to the nail bed. In cases of superficial white onychomycosis, the whitish superficial area on the nail plate was removed using a scalpel blade or surgical scissors.
Two samples were taken simultaneously from each patient; sample #1 was sent to the microbiology department for fungal culture, and sample #2 was used for the rapid antigen detection test. According to the technical specifications of the Diafactory® kit, a sample of, at least, 1mg is required, which in clinical practice is equivalent to a sample of 0.1 mm2 up to 0.5mm2.
The sample was placed in a plastic test tube. The extraction solution was added until it reached the marked limit on the tube, which equals 250μL up to 500μL. The tube was then shaken with the stirring rod 10-20 times and left to stand for 5minutes. Finally, the liquid was poured into the well of the plate containing the test strip, and the result was observed after 5minutes at room temperature (0-37°C).
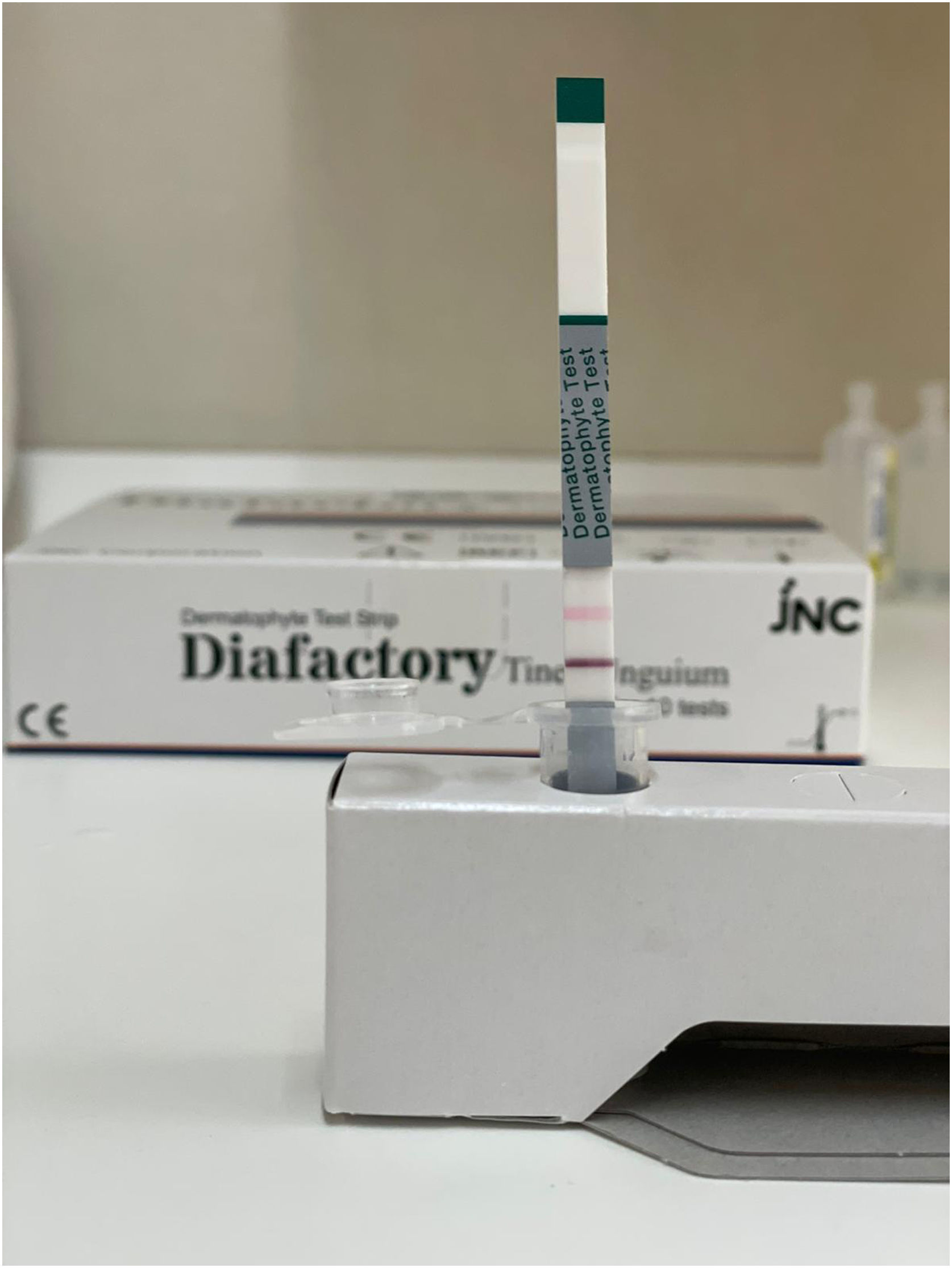
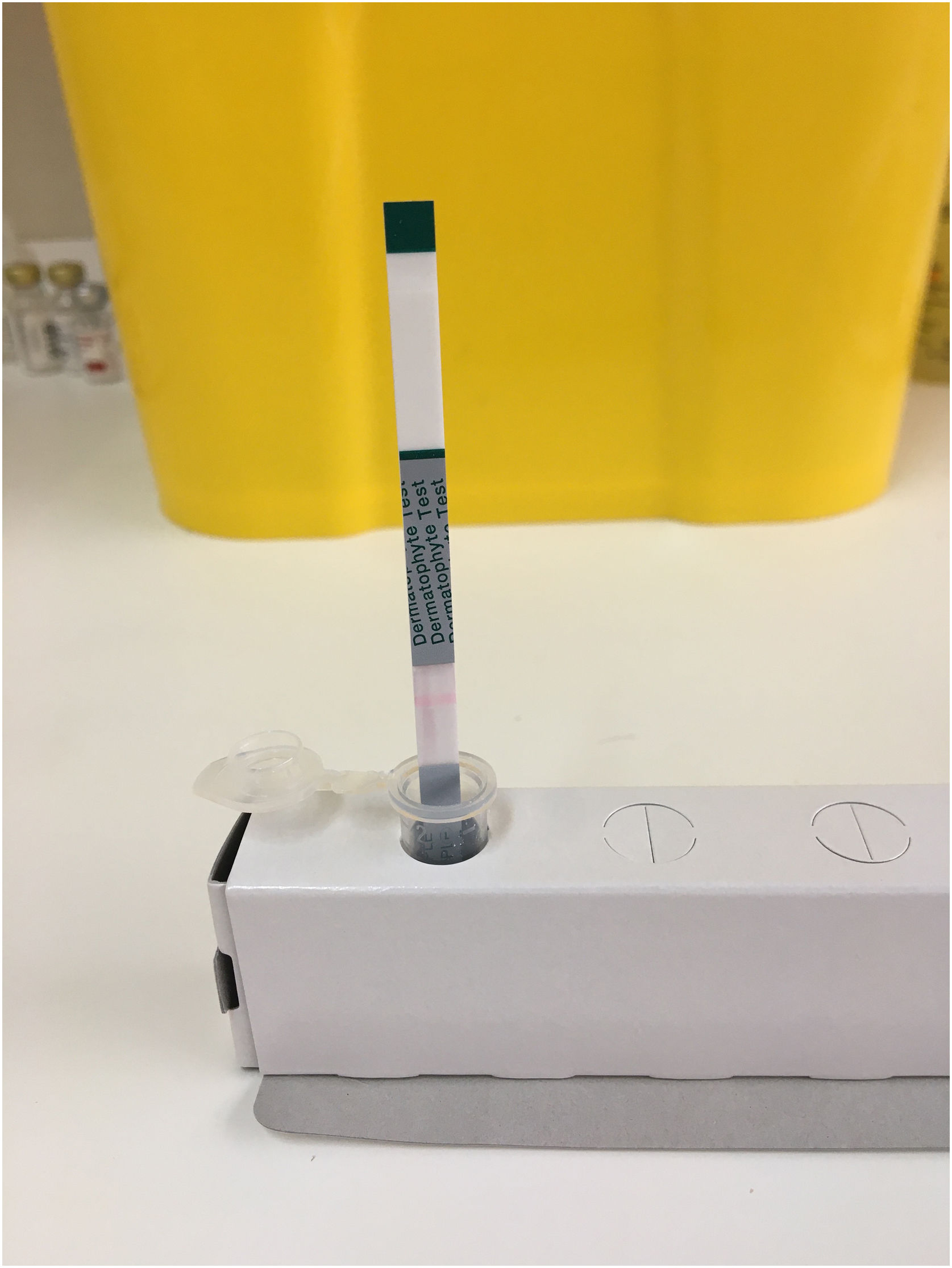
When the sample is absorbed by the test strip, if there is a dermatophyte-derived antigen, it reacts with the anti-dermatophyte antibody—marked with colloidal gold—forming an immune complex. This immune complex continues to move along the test strip and is captured by the monoclonal anti-dermatophyte antibody, becoming immobilized on the strip where a purple line appears, derived from colloidal gold particles, in the case of a positive reaction (Fig. 1). If there are no dermatophyte-derived antigens in the sample, no immune complex is formed, the marked antibody continues along the strip without being captured, and no purple line forms, indicating a negative reaction (Fig. 2).
Regardless of whether the reaction is positive or negative, the sample solution, as it passes over the control line, creates a pink line due to eosin approaching neutral pH when it comes into contact with moisture. If control line does not turn pink, the result is invalid due to a defective test or unsuitable conditions (Fig. 3).
Patients with a positive result in the rapid antigen test began antifungal treatment, and most were discharged from the dermatology department, with follow-up conducted later in primary care. Patients with a negative result in the rapid antigen test were called for 3 consecutive weeks to repeat sample collection for serial cultures. They were then evaluated in a 4th hospital visit, 4 weeks after the last culture, to assess the results and start the required treatment.
This study complies with the 1975 Declaration of Helsinki and its subsequent amendments. Approval for this study was obtained from Hospital Universitario de Fuenlabrada ethics committee (protocol code: EC1749).
ResultsA total of 333 samples were analyzed (129 men [38.7%] and 204 women [61.3%]), with ages ranging from 4 up to 91 years, a mean age of 51.8 years, and a median age of 51 years.
There was a total of 99 cultures with a positive result; 73 of these tested positive for dermatophytes, with T. rubrum being identified in 63 cases, T. interdigitale in 9 cases, and E. floccosum in one case (Table 1). The remaining 26 cultures tested positive for non-dermatophyte fungi; 2 cases of Acremonium sp., 22 cases of Candida sp., 1 case of Rhodotorula sp., and 1 case of Scopulariopsis brevicaulis were identified (Table 2).
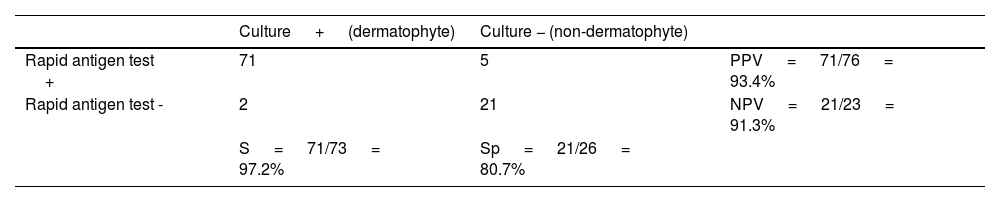
Of the 73 positive dermatophyte cultures, 71 had a positive rapid antigen test, yielding a sensitivity rate of 97.2% for positive cultures. On the other hand, 21 out of the 26 positive cultures for non-dermatophyte fungi had a negative rapid antigen test, showing a specificity rate of 80.7%. The PPV was 93.4%, and the NPV was 91.3% (Table 3).
Sensitivity (S), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) of the rapid antigen detection test vs culture for diagnosing onychomycosis.
| Culture + (dermatophyte) | Culture − (non-dermatophyte) | ||
|---|---|---|---|
| Rapid antigen test + | 71 | 5 | PPV = 71/76 = 93.4% |
| Rapid antigen test - | 2 | 21 | NPV = 21/23 = 91.3% |
| S = 71/73 = 97.2% | Sp = 21/26 = 80.7% |
Analysis of the 234 negative cultures showed that 51 of them had a positive rapid antigen test, so serial cultures were not performed on these patients. Considering fungal culture as the reference technique, these samples would correspond to false negatives. After reviewing the health records of these patients, it was confirmed that all of them were prescribed topical or oral antifungal treatment. Only 16 (31.37%) were reviewed in consultation, and of these, 15 showed clinical improvement after starting therapy. The only patient who did not improve admitted to not having followed the therapy. The 35 patients who were not reassessed did not consult again at the hospital or in primary care for this reason during the 12 months following the dermatology visit.
DiscussionThe high prevalence of onychomycosis in our region, along with the delay in obtaining diagnostic confirmation through culture, leads to significant health care resource expenditure, forcing patients to visit health care centers multiple times before starting therapy. The implementation of the rapid antigen detection test in Europe is an alternative to expedite diagnosis and treatment initiation for these patients.
The rapid dermatophyte antigen detection test uses antibodies against Trichophyton species. The antibody was generated by immunizing mice with an antigen derived from T. rubrum. The epitope for the antibody is a sugar common to the 8 dermatophyte species (T. rubrum, T. interdigitale, T. mentagrophytes, T. violaceum, T. tonsurans, M. canis, M. gypseum, and E. floccosum).13 However, this diagnostic technique has not been designed to identify other species such as Candida, Malassezia, or Scopulariopsis, or other bacterial strains. Although it may show a positive reaction with some species of Aspergillus, Penicillium, and Fusarium, these are not part of the resident microbiota of healthy nails.13,14
We evaluated the rapid antigen detection test for dermatophytes in nail samples from hands and feet. Our examination of 333 samples revealed that the sensitivity and specificity rates of the technique vs culture were 97.2% and 80.7%, respectively.
To date, studies have evaluated the rapid dermatophyte antigen detection test using direct observation as the reference technique, achieving sensitivity rates of 85.4% up to 100% and specificity rates of 34.6% up to 100%.15,16 The first study comparing the rapid dermatophyte antigen detection test with fungal culture as the reference technique, with a slightly smaller sample size than our study, demonstrated a sensitivity rate of 96.07% and a specificity rate of 72.4%.1 The specificity achieved in our study is higher.
Since serial cultures were not performed on samples with a positive antigen test, those with negative cultures could be due to considering the single culture as the reference technique, given that the sensitivity of fungal culture is not 100%. By performing only 1 sample collection for fungal culture instead of serial cultures, it is possible that if a 2nd or 3rd culture had been performed, a few would have tested positive. The clinical improvement observed in patients reviewed after antifungal treatment supports that their nail changes were indeed onychomycosis, as indicated by their positive antigen test.
A comparison between this rapid dermatophyte antigen detection test and PCR is necessary, as PCR achieves excellent sensitivity and specificity and could serve as a true gold standard, although it is not the diagnostic technique we routinely use.
Despite the advantages of this rapid diagnostic technique, it does not eliminate the need to send a sample fragment for fungal culture. This is how we can diagnose cases of onychomycosis due to non-dermatophyte fungi, such as Candida sp, which has a faster growth rate against dermatophytes, potentially growing in days rather than weeks. Maintaining the culture is an advantage when conducting epidemiological studies, prevalence research, or resistance studies to treatments.
The cost of the antigen test is not significant vs the reduction in hospital visits, human resources, and time for nursing and medical staff, which would lead to a clear decrease in the economic impact of this disease. Furthermore, we believe this technique could be very useful in primary care, avoiding the need for specialized care in most cases, as long as a parallel fungal culture is performed. Future research in this area would be beneficial to implement this new diagnostic technique in other health care settings.
ConclusionsOur findings indicate that the efficacy of the rapid dermatophyte antigen detection test is comparable to that of a single culture for the detection of dermatophytes. This diagnostic test provides a quick and reliable diagnosis of dermatophytosis in nails. Its implementation does not require sophisticated equipment or specialized knowledge and allows results to be obtained within minutes. The test enables early initiation of antifungal treatment, and in the case of a negative result, reduces the number of unnecessary antifungal treatments.
We suggest that the rapid dermatophyte antigen detection test is an effective technique for screening onychomycosis, which could prevent patients from multiple additional hospital visits and enable early treatment initiation.
FundingNone declared.
Conflicts of interestNone declared.