The term deep morphea refers to a localized scleroderma in which dermal sclerosis and inflammation extend into the deep dermis, subcutaneous tissue, or even the fascia and superficial muscle.1 In contrast, eosinophilic fasciitis (EF) is characterized by the acute onset of symmetric edema and erythema of the extremities, later replaced by indurated plaques with an “orange peel” appearance as deep sclerosis develops.2 EF frequently shows specific analytical and histologic features, including peripheral eosinophilia, elevated erythrocyte sedimentation rate, hypergammaglobulinemia, and eosinophilic infiltration of the fascia.1,2 However, a significant overlap exists between morphea and EF, and EF is considered a severe clinical presentation within the morphea spectrum.2,3 Drug-induced morphea and EF have been only rarely reported.
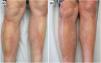
A 55-year-old man presented with a 4-month history of bilateral cutaneous lesions and progressive extension, located in the pretibial regions. His chronic drugs included omeprazole, calcifediol, vitamin B12, etoricoxib, paracetamol/tramadol, lorazepam, and escitalopram. Additionally, he had initiated simvastatin 20mg approximately 2 weeks prior to the onset of lesions. Physical examination revealed the presence of 2 indurated, whitish, alopecic plaques on both pretibial areas with a striking violaceous inflammatory border (Fig. 1a). The patient was otherwise asymptomatic, with no systemic signs.
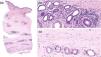
Histopathologic evaluation of a deep skin biopsy (hematoxylin–eosin) showed marked thickening of the dermis and subcutaneous tissue due to hyalinized collagen deposition, along with a lymphoplasmacytic inflammatory infiltrate (Fig. 2). Magnetic resonance imaging demonstrated soft tissue edema without fascial or muscular involvement. Complete blood count revealed neither eosinophilia nor hypergammaglobulinemia, while autoimmune testing showed ANA positivity (1:160) with a fine-speckled nuclear pattern. Borrelia serology tested negative. Based on these findings, a diagnosis of deep morphea was established, and subcutaneous methotrexate (MTX) 10mg/week was initiated. Considering the temporal relationship between simvastatin initiation and symptom onset, the drug was discontinued. The patient experienced progressive clinical improvement 6 weeks into therapy, reaching a maximum MTX dose of 15mg/week, with a marked reduction in induration followed by postinflammatory hyperpigmentation (Fig. 1b). Rosuvastatin was later introduced for dyslipidemia management. Three years after initiating MTX 10mg/week, the patient maintains good disease control, with only mild residual induration and no signs of activity.
Histopathologic images. (a) Low-power view showing thickened dermis due to collagen deposition extending into the deep portion of the biopsy (H&E, ×1). (b) Detail of a group of eccrine glands with atrophy and absence of surrounding adipose tissue, replaced by collagen, along with numerous plasma cells (H&E, ×20). (c) Vessels with markedly thickened walls and pronounced dermal homogenization (H&E, ×20).
A wide range of drug classes has been associated with the induction or exacerbation of connective tissue diseases.4 Specifically, agents such as bleomycin, d-penicillamine, vitamin K1, balicatib, taxanes, and more recently immune checkpoint inhibitors such as nivolumab and pembrolizumab have been involved in the development of morphea.5,6 Although no data link statin use to morphea, an association between HMG-CoA reductase inhibitors and EF has been described (Table 1).7–9 It has been proposed that T-cell differentiation toward a Th2 profile may explain the triggering or exacerbation of certain autoimmune diseases by these drugs, including EF.9 Indeed, a Th2 predominance with elevation of profibrotic cytokines such as IL-4 and IL-13 has been documented in patients with morphea, particularly in the fibrotic phase of the disease.2
Published cases of sclerosing cutaneous disorders within the eosinophilic fasciitis–morphea spectrum associated with statin use.
| Authors | Drug | Disease | Latency period | Statin discontinued | Analytical abnormalities | Systemic treatment |
|---|---|---|---|---|---|---|
| Choquet-Kastylevsky et al.7 | Simvastatin | Eosinophilic fasciitis | 18 months | Yes | Eosinophilia (1.4×109/L); Elevated ESR (36mm); Elevated CRP (23mg/L); Polyclonal hypergammaglobulinemia | Prednisone |
| De Giovanni et al.8 | Atorvastatin | – | 1 week | Yes | Eosinophilia (1.4×109/L); Elevated aldolase (9U/L) | Prednisolone, CsA, PUVA-bath, deflazacort, MTX |
| Serrano-Grau et al.9 | Simvastatin | Eosinophilic fasciitis | 3 weeks | Yes | Mild eosinophilia (0.65×109/L); Elevated ESR (27mm) | Prednisone and MTX |
| Álvarez-Salafranca et al. | Simvastatin | Deep morphea | 2 weeks | Yes | Elevated ESR (27mm); Mildly elevated CRP (10mg/L); ANA+ (1:160) | MTX |
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CsA, cyclosporine A; PUVA, psoralen plus UVA; MTX, methotrexate; ANA, antinuclear antibodies.
Regarding drug-induced morphea, discontinuation of the causative agent may not be sufficient to achieve complete remission, making systemic treatment necessary.10 In such cases, the drug may act as a trigger for a previously latent or subclinical condition.5 Among the few reported cases of statin-associated EF, all patients required systemic corticosteroids and/or methotrexate in addition to drug withdrawal, with progressive improvement (Table 1).
Since morphea and EF may be considered part of a shared clinical spectrum, it is plausible that a common pathogenic mechanism may underlie drug-induced cases of both entities. To our knowledge, this is the first case ever reported of morphea associated with statin therapy. As statins are widely prescribed, this association may be coincidental; however, dermatologists should remain aware of the potential for sclerosing cutaneous disorders due to this class of drugs.
Conflict of interestThe authors declare that they have no conflict of interest.