Acne is a chronic inflammatory disease, in which different events intervene in its pathogenesis, one of which is Cutibacterium acnes (C. acnes). Resistance of this bacteria to different antimicrobials used in treatment has been described in different regions of the world. The purpose of the study is to estimate the resistance of C. acnes to cyclins in patients with moderate and severe acne over 18 years of age.
Materials and methodsAn analytical cross-sectional study was carried out. Samples were taken from inflammatory lesions with a comedone extractor. The content of the samples was incubated in an anaerobic atmosphere to grow C. acnes. Finally, the susceptibility of C. acnes to tetracycline, minocycline and doxycycline was determined.
ResultsSamples were taken from 147 patients, of which 129 showed growth of C. acnes, finding that 5.43% of the strains were resistant to tetracycline, 5.43% to doxycycline, 0.78% to minocycline and cross resistance between doxycycline and tetracycline in all the cases. An association was found between resistance and being 25 years of age or older. No association was found with the prior use of antibiotics, a history of misuse of oral or topical antibiotics, and other demographic and clinical characteristics evaluated.
ConclusionsThe resistance found of C. acnes to cyclines was lower than that reported in other studies. Although no relationship was found with the previous use of antibiotics, it is a factor described in previous studies, which is why the proper use of antibiotics is imperative to avoid the appearance of resistance.
El acné es una enfermedad inflamatoria crónica, en cuya patogenia intervienen diferentes actores, siendo Cutibacterium acnes (C. acnes) uno de ellos. La resistencia de esta bacteria a los diferentes fármacos antimicrobianos utilizados en su tratamiento ha sido descrita en diferentes regiones del mundo. El objetivo de este estudio fue estimar la resistencia de C. acnes a las ciclinas en pacientes mayores de 18 años con acné de moderado a severo.
Materiales y métodosSe realizó un estudio transversal analítico. Se obtuvieron muestras de las lesiones inflamatorias con un extractor de comedones. Se incubó el contenido de las muestras en ambiente anaeróbico para aislar C. acnes. Por último se estimó la susceptibilidad de C. acnes a la tetraciclina, minociclina y doxiciclina.
ResultadosSe obtuvieron muestras de 147 pacientes, observándose crecimiento de C. acnes en 129 de ellos, y encontrándose que el 5,43% de las cepas era resistente a tetraciclina, el 5,43% a doxiciclina, el 0,78% a minociclina, y una resistencia cruzada entre doxiciclina y tetraciclina en todos los casos. Se encontró una asociación entre resistencia y edad igual o superior a 25 años. No se encontró asociación con el uso previo de antibióticos, historia de mal uso de antibióticos orales o tópicos, y otras características demográficos y clínicas evaluadas.
ConclusionesLa resistencia a las ciclinas encontrada en C. acnes fue menor que la reportada en otros estudios. Aunque no se encontró relación con el uso previo de antibióticos, se trata de un factor descrito en estudios previos, por lo que el uso correcto de los mismos es imperativo para evitar la aparición de resistencia.
Acne is a chronic inflammatory disease, clinically characterized by the appearance of non-inflammatory (open or closed comedones) and inflammatory lesions (papules, pustules and nodules), and various degrees of scarring1–3. It is the most common dermatological pathology worldwide, affecting mainly more than 80% of adolescents1,4–8. It is a multifactorial disease1 whose main pathophysiological mechanisms that are associated with the development of acne are: increased sebum production by the sebaceous glands, alteration in the keratinization process, follicular colonization by Cutibacterium acnes, and release of inflammatory mediators in the skin1,2,4.
Cutibacterium acnes (C. acnes) previously named Propionibacterium acnes, is a strict anaerobic gram-positive bacillus that is part of the commensal flora of the skin, predominantly in the sebaceous glands, representing between 20–70% of the microflora9,10. The presence of C. acnes is associated with the induction and amplification of the inflammatory response of the pilosebaceous unit, and the formation of biofilm, which results in follicular hyperkeratinization, lipogenesis, and microcomedogenesis, which in turn, generates a microenvironment conducive to growth and expansion of the microorganism. The uncontrolled inflammatory response clinically leads to more severe acne skin lesions9.
Antibiotic treatment is associated with two important benefits in the management of acne: first, by suppressing the growth of C. acnes, the production of inflammatory mediators synthesized and released by this microorganism is decreased and, on the other hand, the inflammatory response caused by the presence of the microorganism is decreased11. Among the oral antibiotics indicated for acne treatment are cyclines, trimethoprim/sulfamethoxazole3,4 and macrolides 1,3,4,11. In Colombia the use of oral antibiotics is indicated in the treatment of moderate to severe acne, with tetracyclines being the first line of treatment3,4.
The frequent use but mainly the misuse of antibiotics in different infectious pathologies has led to the development of bacterial resistance, becoming a public health problem worldwide. In fact, according to the latest report from the World Health Organization, there are high rates of resistance for the microorganisms that cause the most frequent infections18. Specifically in acne, an increasing resistance of C. acnes to different oral antibiotics has been reported in recent years10.
The first resistant strain of C. acnes was described in 1979, and since then, different publications have reported resistance data that increased from 34.5% in 1991 to 64% in 1997(12,13); Although a decrease was observed in 1999 to 50.5%, in 2000 an increase to 55% was reported10, with variations between countries in the reported resistance rates5,12–16.
Resistance of C. acnes is mainly associated with a poorer response rate to treatment and a higher number of exacerbations in patients with acne4. However, different studies have shown that in addition to therapeutic failure, antibiotic resistance can be accompanied by genetic transfer of this mechanism to other skin pathogens, such as Staphylococcus aureus or Streptococcus pyogenes, increasing the rates of antibiotic resistance of these microorganisms17.
In Colombia, a study was previously carried out in 2005–2006 involving two dermatological centers. The objective of which was to determine the antimicrobial susceptibility of C. acnes to the antibiotics commonly used in acne (erythromycin, clindamycin, tetracycline, doxycycline, minocycline), finding that 40% of the isolated C. acnes were resistant to at least one of the antibiotics tested and 63% had received antibiotic treatment in the last 6 months. The risk factor that was associated with greater antibiotic resistance was a history of antibiotic use, since a 46% resistance was demonstrated among patients who reported this history, however resistance was demonstrated even in patients without a history antibiotics usege showing resistance of 28%5.
Taking into account that in the dermatological center of the study, acne is the main reason for consultation, with about 9 thousand new diagnoses per year18, and that there are few surveillance studies of resistance of C. acnes to cyclines in Colombia, it is proposed to estimate the frequency of cycline resistance and identify associated factors, among our patients.
Materials and methodsAn cross-sectional analytical study was carried out. Two expert dermatologists at the institutional acne clinic selected patients over 18 years of age with moderate to severe acne from February 2017 to May 2018. Patients with any of the following criteria were excluded: severe acne requiring isotretinoin management oral, topical or oral antimicrobial treatment in the last 3 months and pregnancy.
Samples were taken from the face or trunk from inflammatory lesions (papule or pustule) with a comedone extractor. The contents of the samples were placed in a tube with thioglycollate broth enriched with hemin and menadione, then seeded in two boxes with Schaedler agar and incubated in an anaerobic atmosphere at 37 °C for 4–6 days. C. acnes was identified by the characteristics of its colonies, gram staining, and with the help of a stereomicroscope the colonies were obtained for subculture in order to have pure bacterial growth. Bacterial identification was performed with the Thermo Scientific™ RapID™ ANA II System kit for anaerobes. Strain ATCC 11827 was used as a control for the tests. To determine the susceptibility of C. acnes to tetracycline, minocycline and doxycycline, the epsilometer E test® BioMérieux range between 0.016 a 256 ug/mL was used. The protocol for the isolates and subsequent subcultures were made according to the techniques recommended in The Clinical & Laboratory Standards Institute (CLSI) M100 and CLSI M11-A8 guidelines. The E tests were performed on Wilkins Chalgren agar. Isolates were considered resistant for all antibiotics with MIC of >ó = 16 ug/mL19,20.
Patients were selected by convenience if they met the eligibility criteria and a sample size was estimated using the Epi Info™ 7 software, taking into account an expected frequency of resistance of 9%, Confidence Interval of 95%, error of 5%, statistical power of 80% and losses of 10%. In the statistical analysis, the categorical variables were expressed as frequencies, and measures of central tendency and dispersion were performed for the quantitative variables. Bivariate analyzes were performed to search for an association between resistance of C. acnes to tetracyclines (dependent variable) and the sociodemographic and clinical factors of interest (independent variables). The null hypothesis was established that there are no differences between the proportion of global resistance between the groups with the factors analyzed. As a criterion for rejection of the null hypothesis, a level of statistical significance less than 0.05 was defined. The statistics used for the differences in proportions were the Z test and the chi-square test. For the resistances, intervals were estimated at 95% reliability by the exact binomial method. A stratified Mantel–Haenszel analysis was performed to evaluate confounding factors and a multivariate analysis was performed using the logistic model, whose dependent variable was global resistance to cyclines, for which this variable had three categories (sensitive, intermediate and resistant) it was re-categorized into two: presence or absence of resistance to cyclins. The analyzes were performed using the Stata 13 statistical package.
The study was conceived in accordance with the considerations established in the Declaration of Helsinki and complies with the guidelines of the Colombian regulations set forth in Resolution 008430 of 1993 of the Ministry of Health. It was classified as an investigation with minimal risk as defined in the 11th article of the aforementioned resolution21. All the patients agreed to participate in the study voluntarily, the risks of taking the sample were explained, with subsequent signing of the informed consent. There were no adverse events as a result of sampling or attention. The study was approved by the ethics committee of the Federico Lleras Acosta Dermatological Center and the National University of Colombia.
Results147 samples of skin lesions were collected from patients with acne vulgaris, of which 14 were not isolated C. acnes and in 3 were Propionibacterium spp. Finally, 129 patients were included for the analysis due to the growth of C. acnes.
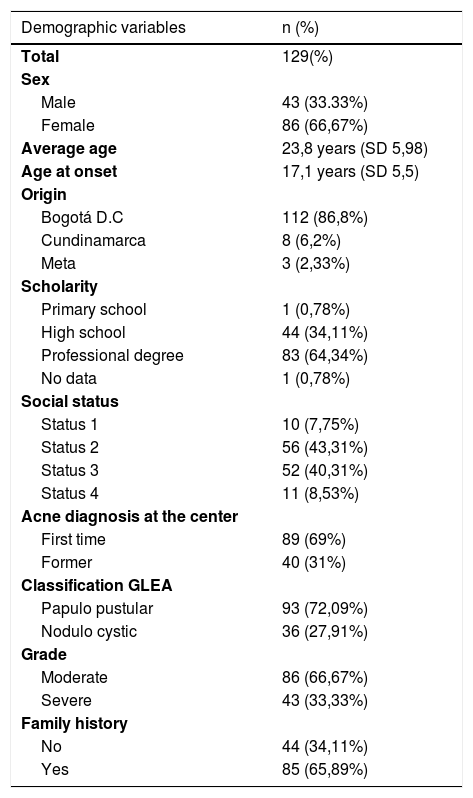
In the demographic characteristics of the patients, it was found that the female sex was 66.7%, the average age of the sample was 23.8 years, with a range of 18–49 years, and the mean age for disease onset was 17.1 years. Bogotá was the municipality from which the most patients were included with 86.8%, followed by the departments of Cundinamarca and Meta. Most of the patients had completed higher education (64%), were low-income (83%), and 69% of the patients were new to the institution. The population structure of the sample coincides with the profile of patients treated at the institution18, (Table 1).
Variables demográficas.
| Demographic variables | n (%) |
|---|---|
| Total | 129(%) |
| Sex | |
| Male | 43 (33.33%) |
| Female | 86 (66,67%) |
| Average age | 23,8 years (SD 5,98) |
| Age at onset | 17,1 years (SD 5,5) |
| Origin | |
| Bogotá D.C | 112 (86,8%) |
| Cundinamarca | 8 (6,2%) |
| Meta | 3 (2,33%) |
| Scholarity | |
| Primary school | 1 (0,78%) |
| High school | 44 (34,11%) |
| Professional degree | 83 (64,34%) |
| No data | 1 (0,78%) |
| Social status | |
| Status 1 | 10 (7,75%) |
| Status 2 | 56 (43,31%) |
| Status 3 | 52 (40,31%) |
| Status 4 | 11 (8,53%) |
| Acne diagnosis at the center | |
| First time | 89 (69%) |
| Former | 40 (31%) |
| Classification GLEA | |
| Papulo pustular | 93 (72,09%) |
| Nodulo cystic | 36 (27,91%) |
| Grade | |
| Moderate | 86 (66,67%) |
| Severe | 43 (33,33%) |
| Family history | |
| No | 44 (34,11%) |
| Yes | 85 (65,89%) |
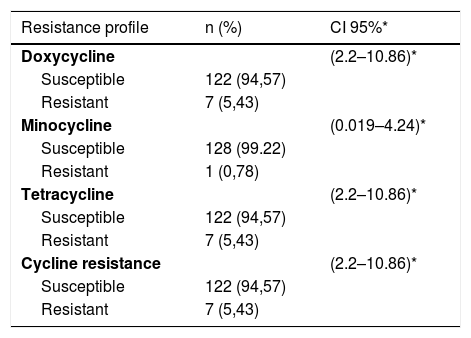
With regard to the clinical characteristics, and according to the classification of the Latin American Group for the Study of Acne (GLEA), it was found that papule-pustular acne was the most frequent with 93%, and in terms of the degree of severity, the moderate was the one that more contributed with 86%. The most common location of acne was on the face and trunk in 60%, followed by unique involvement of face in 36%. The oral antibiotic more frequently before entering the study was doxycycline with 15.5%, of the topical medications, benzoyl peroxide and clindamycin were previously used in 14.7 and 4%, respectively, (Table 1). The lowest resistance found was to minocycline with 0.78%, followed by doxycycline and tetracycline with 5.43% each, (Table 2 shows the resistances).
Resistance profile.
| Resistance profile | n (%) | CI 95%* |
|---|---|---|
| Doxycycline | (2.2–10.86)* | |
| Susceptible | 122 (94,57) | |
| Resistant | 7 (5,43) | |
| Minocycline | (0.019–4.24)* | |
| Susceptible | 128 (99.22) | |
| Resistant | 1 (0,78) | |
| Tetracycline | (2.2–10.86)* | |
| Susceptible | 122 (94,57) | |
| Resistant | 7 (5,43) | |
| Cycline resistance | (2.2–10.86)* | |
| Susceptible | 122 (94,57) | |
| Resistant | 7 (5,43) |
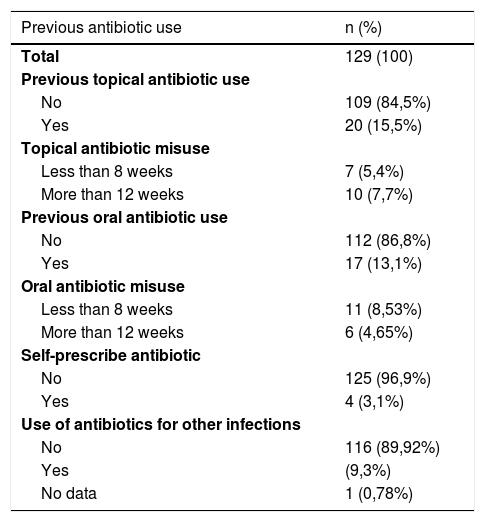
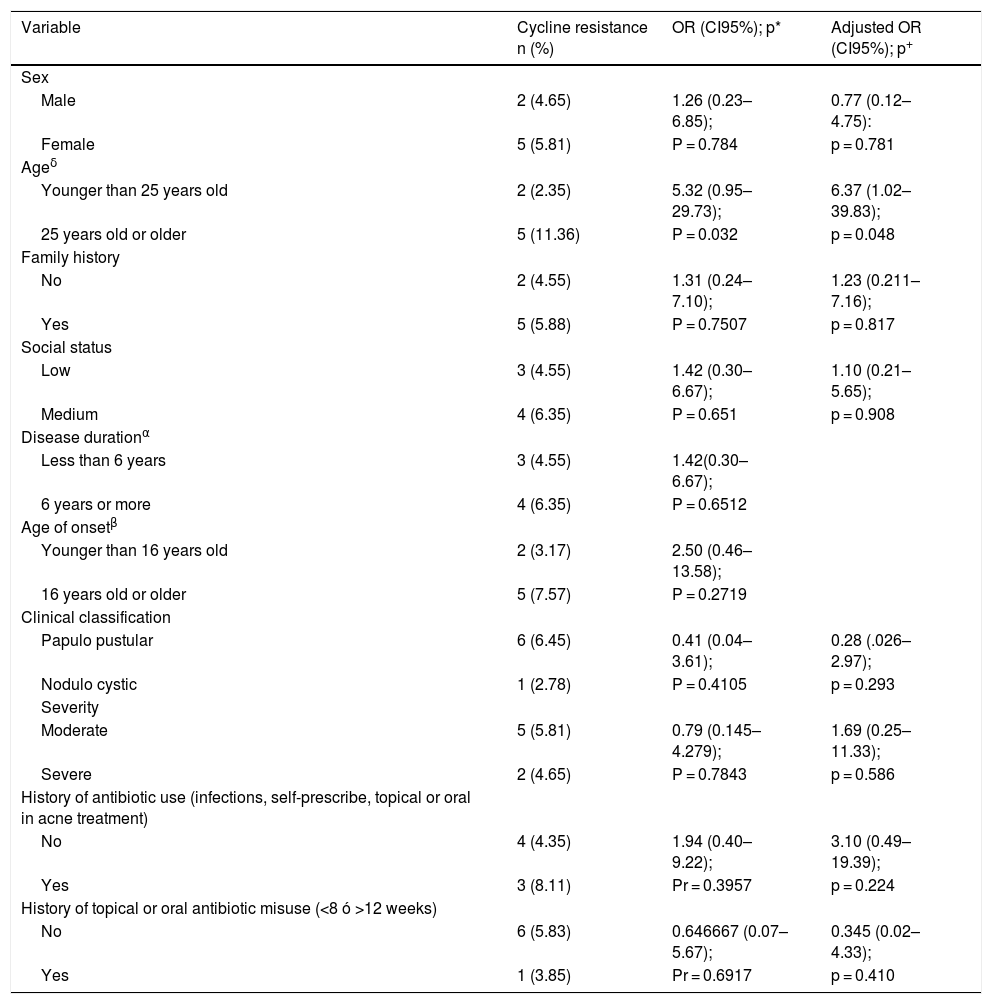
Among the possible risk factors evaluated for resistance, the history of topical antibiotic use was 15.5%, the history of misuse of topical antibiotics (<8 weeks or >12 weeks) was 13.1%, the history of oral antibiotic use was 13.1%, the history of oral antibiotic misuse (<8 weeks or >12 weeks) was 13.1%, self-medicated antibiotics was 3.1%, and the use of antibiotics for other infections was 9.3%, (Table 3). The evaluated factors were regrouped to establish associations with the outcome of resistance, which was done with age, socioeconomic stratum, duration of the disease, age of onset of the disease, history of antibiotic use and history of both oral and topical misuse, (Table 4).
Previous antibiotic use.
| Previous antibiotic use | n (%) |
|---|---|
| Total | 129 (100) |
| Previous topical antibiotic use | |
| No | 109 (84,5%) |
| Yes | 20 (15,5%) |
| Topical antibiotic misuse | |
| Less than 8 weeks | 7 (5,4%) |
| More than 12 weeks | 10 (7,7%) |
| Previous oral antibiotic use | |
| No | 112 (86,8%) |
| Yes | 17 (13,1%) |
| Oral antibiotic misuse | |
| Less than 8 weeks | 11 (8,53%) |
| More than 12 weeks | 6 (4,65%) |
| Self-prescribe antibiotic | |
| No | 125 (96,9%) |
| Yes | 4 (3,1%) |
| Use of antibiotics for other infections | |
| No | 116 (89,92%) |
| Yes | (9,3%) |
| No data | 1 (0,78%) |
Resistance associations.
| Variable | Cycline resistance n (%) | OR (CI95%); p* | Adjusted OR (CI95%); p+ |
|---|---|---|---|
| Sex | |||
| Male | 2 (4.65) | 1.26 (0.23–6.85); | 0.77 (0.12–4.75): |
| Female | 5 (5.81) | P = 0.784 | p = 0.781 |
| Ageδ | |||
| Younger than 25 years old | 2 (2.35) | 5.32 (0.95–29.73); | 6.37 (1.02–39.83); |
| 25 years old or older | 5 (11.36) | P = 0.032 | p = 0.048 |
| Family history | |||
| No | 2 (4.55) | 1.31 (0.24–7.10); | 1.23 (0.211–7.16); |
| Yes | 5 (5.88) | P = 0.7507 | p = 0.817 |
| Social status | |||
| Low | 3 (4.55) | 1.42 (0.30–6.67); | 1.10 (0.21–5.65); |
| Medium | 4 (6.35) | P = 0.651 | p = 0.908 |
| Disease durationα | |||
| Less than 6 years | 3 (4.55) | 1.42(0.30–6.67); | |
| 6 years or more | 4 (6.35) | P = 0.6512 | |
| Age of onsetβ | |||
| Younger than 16 years old | 2 (3.17) | 2.50 (0.46–13.58); | |
| 16 years old or older | 5 (7.57) | P = 0.2719 | |
| Clinical classification | |||
| Papulo pustular | 6 (6.45) | 0.41 (0.04–3.61); | 0.28 (.026–2.97); |
| Nodulo cystic | 1 (2.78) | P = 0.4105 | p = 0.293 |
| Severity | |||
| Moderate | 5 (5.81) | 0.79 (0.145–4.279); | 1.69 (0.25–11.33); |
| Severe | 2 (4.65) | P = 0.7843 | p = 0.586 |
| History of antibiotic use (infections, self-prescribe, topical or oral in acne treatment) | |||
| No | 4 (4.35) | 1.94 (0.40–9.22); | 3.10 (0.49–19.39); |
| Yes | 3 (8.11) | Pr = 0.3957 | p = 0.224 |
| History of topical or oral antibiotic misuse (<8 ó >12 weeks) | |||
| No | 6 (5.83) | 0.646667 (0.07–5.67); | 0.345 (0.02–4.33); |
| Yes | 1 (3.85) | Pr = 0.6917 | p = 0.410 |
*OR (odds ratio), p = probability of rejection with the Z test for difference of proportions; +Adjusted OR and probability of rejection with the logistic model; αThe duration of the disease was defined as a cut-off point of 5 years because it is the median; βThe age of disease onset was defined as 16 years because it is the median; δIn the age of the patient, the cut-off of 24 years was used, because using the median in 22 years as a criterion prevented the calculation of the OR because there were zeros in the resistance cells.
In the bivariate analysis, it was found that those over 25 years of age are associated with resistance to cyclines, (Table 4). With the rest of the factors evaluated, no significant associations were found. To define possible confounding factors that were affecting the association between the age variable with resistance to cyclines, a Mantel-Haenszel stratified analysis was performed, generally obtaining very wide confidence intervals for the stratified OR. In this analysis, the variables age at onset of the disease and duration of the disease significantly modified the crude OR of the relationship studied, for which they were excluded in the multivariate model. With these results, a logistic model was carried out selecting those factors that did not significantly modify the crude OR of the effect of age on resistance when stratifying by the other cofactors. However, the logistic model that showed the best performance in estimating the effect of the age variable on resistance significantly increased the value of statistical significance. Likewise, the post estimation evaluations of the selected model showed that it was not a significant model. The results of the OR adjusted with the confidence intervals and the statistical significance obtained with the model are presented, (the last column of Table 4).
DiscussionIn dermatology, antibiotics are one of the main drugs used in a wide variety of pathologies, but it is mainly use on acne and rosacea, of which two thirds of oral antibiotic formulations are in acne22,23.
Resistance of C. acnes to one or more antibiotics is a problem documented in various studies around the world, with rates that have fluctuated over the years from 34.5% in 1991 to 64% in 1997, 50.5% in 1999 and 55.5% in 2000, with resistance to erythromycin being the most frequent, and the latter being linked to the generation of resistance to clindamycin10.
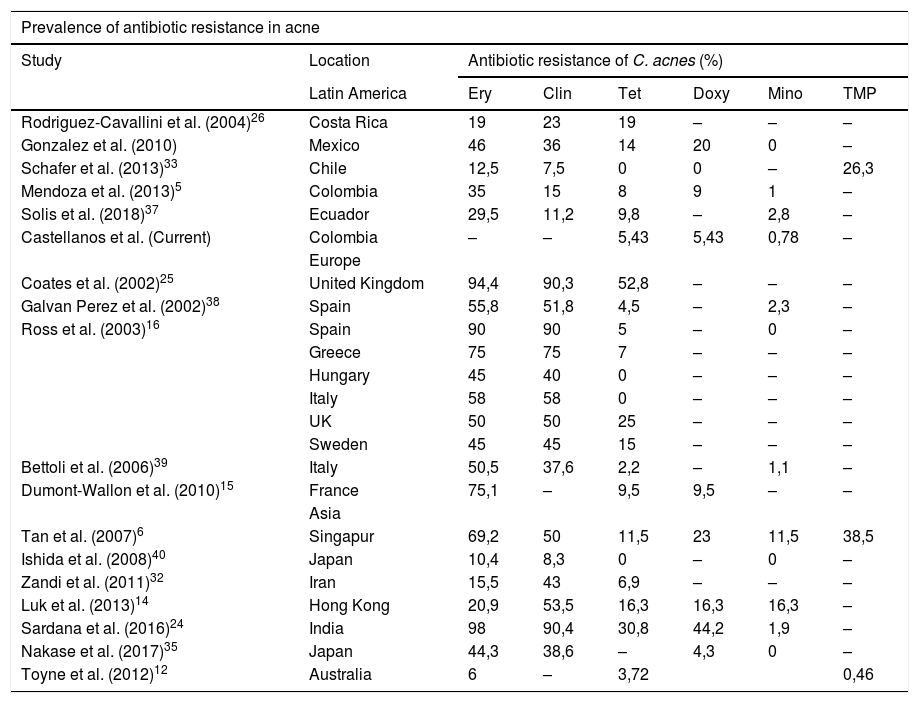
Worldwide, it is well known that India and the United Kingdom have high rates of resistance to tetracycline16,24,25, with highly variable rates for resistance to doxycycline, and low for minocycline, except for that reported in Singapore and Hong Kong6,14. Our findings show consistency with what was found 6 years ago in the country5, where resistance to cyclines is low, especially to minocycline, as in the rest of Latin America, except Costa Rica and Mexico which are the highest of the zone26. Clarifying that, by not evaluating resistance to erythromycin and clindamycin, we will not be able to evaluate all C. acnes resistant to other antibiotic groups (Table 5).
Worldwide acne resistance.
| Prevalence of antibiotic resistance in acne | |||||||
|---|---|---|---|---|---|---|---|
| Study | Location | Antibiotic resistance of C. acnes (%) | |||||
| Latin America | Ery | Clin | Tet | Doxy | Mino | TMP | |
| Rodriguez-Cavallini et al. (2004)26 | Costa Rica | 19 | 23 | 19 | – | – | – |
| Gonzalez et al. (2010) | Mexico | 46 | 36 | 14 | 20 | 0 | – |
| Schafer et al. (2013)33 | Chile | 12,5 | 7,5 | 0 | 0 | – | 26,3 |
| Mendoza et al. (2013)5 | Colombia | 35 | 15 | 8 | 9 | 1 | – |
| Solis et al. (2018)37 | Ecuador | 29,5 | 11,2 | 9,8 | – | 2,8 | – |
| Castellanos et al. (Current) | Colombia | – | – | 5,43 | 5,43 | 0,78 | – |
| Europe | |||||||
| Coates et al. (2002)25 | United Kingdom | 94,4 | 90,3 | 52,8 | – | – | – |
| Galvan Perez et al. (2002)38 | Spain | 55,8 | 51,8 | 4,5 | – | 2,3 | – |
| Ross et al. (2003)16 | Spain | 90 | 90 | 5 | – | 0 | – |
| Greece | 75 | 75 | 7 | – | – | – | |
| Hungary | 45 | 40 | 0 | – | – | – | |
| Italy | 58 | 58 | 0 | – | – | – | |
| UK | 50 | 50 | 25 | – | – | – | |
| Sweden | 45 | 45 | 15 | – | – | – | |
| Bettoli et al. (2006)39 | Italy | 50,5 | 37,6 | 2,2 | – | 1,1 | – |
| Dumont-Wallon et al. (2010)15 | France | 75,1 | – | 9,5 | 9,5 | – | – |
| Asia | |||||||
| Tan et al. (2007)6 | Singapur | 69,2 | 50 | 11,5 | 23 | 11,5 | 38,5 |
| Ishida et al. (2008)40 | Japan | 10,4 | 8,3 | 0 | – | 0 | – |
| Zandi et al. (2011)32 | Iran | 15,5 | 43 | 6,9 | – | – | – |
| Luk et al. (2013)14 | Hong Kong | 20,9 | 53,5 | 16,3 | 16,3 | 16,3 | – |
| Sardana et al. (2016)24 | India | 98 | 90,4 | 30,8 | 44,2 | 1,9 | – |
| Nakase et al. (2017)35 | Japan | 44,3 | 38,6 | – | 4,3 | 0 | – |
| Toyne et al. (2012)12 | Australia | 6 | – | 3,72 | 0,46 | ||
Clin: clindamycin; Doxy: doxycycline; Eri: erythromycin; Mino: minocycline; Tet: tetracycline; TMP: trimethoprim/sulfamethoxazole.
The average age of our population is 24 years old, which is higher than that usually reported between 15 and 20 years27–31, this is due to the fact that the study group does not attend to children. The female predominance of the study has also been shown by other cohorts such as that of Kilkenny et al.30 and other cohorts included in the review by Tan et al.27.
The study population does not have patients with mild disease, and those with moderate disease predominate, contrary to that reported in the world literature where approximately two thirds of patients have mild disease27,28,30, it may be due to the fact that selected patients must have papules, pustules or nodules for sampling, to obtain more material for culture, although this may correspond to a selection bias. An important finding was to find a 100% cross-resistance between resistance to doxycycline and tetracycline, as was also found in the study carried out in France15 and to a lesser extent in other studies14.
The risk factor more frequently associated with antibiotic resistance is the use of topical antibiotics, that was associated with resistant C. acnes in Australia (higher rate of resistance with the use of topical antibiotics (50%) compared to patients with no history of use of antibiotic (3.6%) and the use of oral antibiotics (17.5%))12. The use of oral antibiotics was associated with greater resistance in Mexico (patients with a history of antibiotic treatment presented higher resistance: azithromycin (92.5%), trimethoprim / sulfamethoxazole (77.7%), erythromycin (59.2%), doxycycline (33%) compared to patients without this history azithromycin (72.7%), trimethoprim / sulfamethoxazole ( 59%) and erythromycin (31.8%)). However, a study conducted in Iran did not detect significant differences in antibiotic resistance in patients with and without a history of antibiotic use32. These results indicate that the antibiotics used in acne treatment, may contribute to the formation of resistant strains of C. acnes.
An association between the variables age and resistance was found in the bivariate analysis, understanding that the older the age, the greater the probability of exposure to antibiotics and therefore the greater risk of resistance, as found by Luk et al., and Schafer et al.14,33.
However, no relationship was found with previous exposure to antibiotics, contrary to what was found in many publications in relation to resistance in acne5,12,15,34.
One of the greatest difficulties inherent in retrospective studies is depending on the information that the patient declares. This may explain the paradoxical results that we found with the antecedent variable of misuse of topical or oral antibiotics, in which greater resistance was obtained in those who did not register this antecedent. It was observed that with the variables classification and severity, the groups were not balanced. It is possible that due to the low prevalence of resistance in our population, larger sample size is required to capture the differences in the groups.
C. acnes is a bacterium that plays an important role in the production of acne, antibiotics due to their anti-inflammatory, immunomodulatory and antimicrobial properties have been used to control the disease, but their use has generated the appearance and increase of the resistance of both C. acnes and other microorganisms, and not only in patients, but even in their close contacts and dermatologists35, which invites us to make rational use of antibiotics when strictly necessary, and under adequate prescription. Current dermatology practice tends to look for alternatives to the use and abuse of antibiotics in the treatment of acne. Treatments such as isotretinoin, topical retinoids, and in the future compounds such as 5 alpha-reductase inhibitors are valid alternatives to avoid the use of antibiotics, as well as the management of female acne with oral contraceptives and spironolactone. The results of the study show high sensitivity to the use of cyclines, which may be related to the phylotypes of the studied population and the pattern of use of antibiotics in acne, although the latter could not be corroborated in our institution, because despite adherence to the antibiotic use guidelines of 37.1%, a low resistance was found36. In a near future, the characterization of the phylotypes of C. acnes could answer these questions.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Castellanos Lorduy HJ, Pérez Cely HC, Casadiego Rincón EJ, Henao Riveros SC, Colorado CL, Perfil de resistencia a la tetraciclina de Cutibacterium acnes en pacientes con acné vulgar en un centro dermatológico de Colombia. Actas Dermosifiliogr. 2021;112:873–880.