Leishmaniasis is a chronic disease caused by flagellate protozoa of the genus Leishmania. It is a global disease, but most cases are seen in South America, the Mediterranean, and some areas of Asia and Africa. The 3 main types of leishmaniasis are cutaneous (the most common), mucocutaneous, and visceral (the most severe). Visceral leishmaniasis is also known as kala-azar. Leishmaniasis is diagnosed by demonstrating the presence of Leishmania amastigotes in clinical specimens using direct microscopic examination or molecular analysis. Various treatments exist, although the evidence supporting the options available for cutaneous leishmaniasis is weak. Both the classical presentation of leishmaniasis and our management of the disease have changed in recent decades because of acquired immune deficiency caused by conditions such as human immunodeficiency infection or the use of tumor necrosis factor inhibitors.
La leishmaniasis es una enfermedad crónica causada por un protozoo flagelado perteneciente al género Leishmania. Tiene distribución mundial, aunque la mayoría de casos se agrupan en América del Sur, la cuenca mediterránea y algunas zonas de Asia y África. Existen tres formas fundamentales de enfermedad: cutánea, la más frecuente; mucocutánea; y visceral, también denominada kala-azar, la forma más grave. El diagnóstico se establece con la demostración de la presencia de los amastigotes en muestras clínicas, mediante visión directa al microscopio o mediante técnicas moleculares. Existen múltiples opciones terapéuticas, aunque la evidencia en la que se basa el tratamiento de la leishmaniasis cutánea es débil. Actualmente, las alteraciones de la inmunidad producidas por factores como el VIH o el uso de fármacos anti-TNF han cambiado tanto la forma de presentación de las formas clínicas clásicas como sus tratamientos.
Leishmaniasis is a chronic disease caused by flagellate protozoa of the genus Leishmania, of which there are over 20 species. Leishmania parasites are obligate intracellular parasites transmitted by the bite of infected female sandflies of the Phlebotomus and Lutzomyia genera. Leishmaniasis is essentially a zoonotic disease. The main reservoirs are dogs and rodents, but there are 2 cases for which humans are the main reservoirs: Leishmania donovani and Leishmania tropica.
EpidemiologyThe estimated incidence of leishmaniasis according to the World Health Organization is 700 000 to 1 million cases a year, of which 50 000 to 90 000 correspond to visceral leishmaniasis (VL).1 Approximately 95% of all cutaneous leishmaniasis (CL) cases occur in South America, the Mediterranean Basin, the Middle East, or Central Asia. LV, by contrast, is predominant in Brazil, East Africa, and India. In 2018, over 85% of new CL cases reported to the WHO were from Afghanistan, Algeria, Bolivia, Brazil, Colombia, Iran, Iraq, Pakistan, Syria, and Tunisia, while over 95% of new LV cases were from Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, and Sudan.1 Finally, over 90% of new mucocutaneous leishmaniasis (MCL) cases were reported in 4 countries: Brazil, Bolivia, Ethiopia, and Peru.1
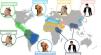
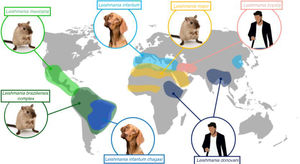
Leishmaniasis has traditionally been classified as Old World or New World depending on its geographic location. Old World leishmaniasis occurs in Asia, Africa, and Europe, and is mostly caused by L tropica, Leishmania major, Leishmania aethiopica, Leishmania infantum, or L donovani. New World leishmaniasis, in turn, occurs in America, and is mostly caused by Leishmania mexicana, Leishmania amazonensis, Leishmania braziliensis, Leishmania panamensis, or Leishmania infantum chagasi (a subspecies of L infantum in the New World, formerly known as Leishmania chagasi).2 The main epidemiological and clinical patterns for most common Leishmania species are shown in Table 1.2–5Fig. 1 shows the geographic distribution of the main species and their principal reservoirs.
Clinical and Epidemiological Characteristics of the Main Species of Leishmania.
| Complex | Main Species | Main Reservoir | Main Geographic Distribution | Most Common Clinical Patterns and Their Main Characteristics | Natural Progression |
|---|---|---|---|---|---|
| Leishmania donovani | L donovani | Human | India, Bangladesh, Ethiopia, Sudan | VL: fever, hepatosplenomegaly, weight loss, anemia | Without treatment, death within 2 y |
| PKDL: macular, papular, or nodular lesions | Resolves spontaneously in up to 85% of cases in Africa, but rarely in Asia | ||||
| Leishmania infantum | Dog, hare | Mediterranean Basin, China | CL: mildly inflammatory solitary nodulesVL: more common in children and immunosuppressed individuals | Tends to resolve spontaneously within a year, conferring immunity | |
| Leishmania infantum chagasi | Dog, fox | Central and South America | |||
| Leishmania tropica | L tropica | Human | Eastern Mediterranean, Middle East, India | CL: <3 dry ulcers, mostly on the head; chronic, recurrent course | Most cases resolve spontaneously within 2 y |
| Leishmania major | Rodents | Africa, Middle East, Central Asia, India, China | LC: multiple moist inflammatory ulcers, most of which progress rapidly | 70% of cases resolve in 4 mo but leave severe scarring | |
| Leishmania aethiopica | Hyraxes (dassie) | Ethiopia, Kenya | LC: localized nodules or ulcers; may result in diffuse involvement | Usually resolves spontaneously with 2-5 years, except for diffuse forms | |
| Leishmania mexicana | L mexicana | Rodents, marsupials | Mexico, Central and South America, Texas | LC: solitary or multiple ulcerated lesions, sometimes with diffuse involvement; responsible for characteristic ulcerated ulcer lesion in gum collectors | >80% of cases resolve spontaneously within 3–4 mo, although some lesions can last up to 20 y |
| Leishmania amazonensis | Rodents, possums | South America | LC: ulcerated lesions, often with diffuse involvementCan progress to MCL | Not well described | |
| Leishmania (Viannia) braziliensis | L braziliensis | Rodents, dogs | Central and South America | LC: ulcerated lesionsMCL: mainly caused by L braziliensis; ulcerated lesions in oral and nasal mucosa; can extend to oropharynx and larynx | Causes serious infections; often associated with satellite lesions, subcutaneous nodules, and enlarged locoregional lymph nodes; 6% of cases resolve spontaneously in 6 mo |
| Leishmania panamensis | Sloths | Panama, Costa Rica, Colombia | CL: superficial ulcers, often with lymphatic spreadMCL: nasopharyngeal involvement | Does not resolve spontaneously. Spreads through lymphatic vessels and can cause mucocutaneous lesions | |
| Leishmania guyanensis | Possums, sloths, and anteaters | South America | LC: multiple ulcers that can spread through the lymphatic system, resulting in a sporotrichoid distributionMCL: cutaneous involvement that can progress to mucocutaneous involvement | Tends to need treatment and recurs frequently; at times, resolves spontaneously within 6 months |
Abbreviations: CL, cutaneous leishmaniasis; MCL, mucocutaneous leishmaniasis; PKDL, post-kala-azar dermal leishmaniasis; VL, visceral leishmaniasis.
In Spain, leishmaniasis is a zoonotic disease endemic to the peninsula and the Balearic Islands. The main causative species in both CL and VL is L infantum, which is transmitted by Phlebotomus perniciosus and Phlebotomus ariasi.6 Dogs are the main reservoir for L infantum, although other reservoirs such as hares7 and rats8 have been described. Apart from its endemic form, leishmaniasis can occur in patients with human immunodeficiency virus (HIV) infection9 or a compromised immune system (e.g., patients being treated with tumor necrosis factor [TNF] inhibitors).10 There have also been epidemic outbreaks, such as the Madrid outbreak that occurred between 2009 and 2013.7 Finally, phenomena such as globalization, international travel, and migration have increased the prevalence of leishmaniasis in developed countries.11
PathogenesisLeishmania promastigotes are injected into human skin by infected female sandflies. Here, through phagocytosis by macrophages, they transform into amastigotes, multiplying inside the cells and infecting other mononuclear phagocytized cells. Sandflies becomes infected by ingesting infected cells while feeding on the host’s blood. Once in the intestine of the sandflies, the amastigotes transform into promastigotes.12 The incubation period of leishmaniasis varies according to the clinical form of disease, but is generally 2 weeks (or less) to 2 months for CL, 3 to 9 months for VL, and over 2 years for MCL.13
The clinical manifestations of leishmaniasis vary according to the species involved14 (Table 1) and the host’s immune response.15 The spectrum of immune response ranges from a strong T-cell response that results in the production of interferon (IFN) γ to a humoral response that produces high antibody levels. Leishmania species are eliminated by IFN-γ-activated macrophages, but cannot be neutralized by antibodies. This is why individuals who mount a strong immune response have lesions with few parasites, while those with a humoral response are unable to control infection. Diffuse CL is caused by uncontrolled infection. It should also be noted that an exaggerated T helper type 1 response and increased expression of CD8+ T cells are associated with more severe forms of disease, such as MCL.15
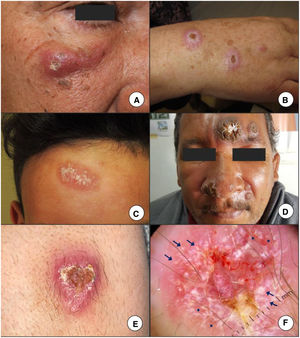
Clinical ManifestationsCutaneous LeishmaniasisCL starts with the formation of a papule at the inoculation site, which is typically located in exposed areas of the body, such as the face or extremities. The papule typically develops into a plaque or a nodule (Fig. 2A-E) with a tendency to ulcerate. Gum collectors (chicleros) in Mexico and Central America, for example, develop a characteristic ulcerated lesion on their ear following infection by L mexicana.16 CL lesions can be single or multiple and infection can spread through the lymphatic system, causing lymph node enlargement, satellite lesions, and even sporotrichoid lesions.17 Atypical forms, such as eczematous, erysipeloid, lupoid, annular, and verrucous lesions, are more common in the New World.18 CL lesions can resolve spontaneously within several months leaving a scar. Some cases, however, become chronic or spread. Chronic recurrent forms are typical in L tropica infections and are characterized by the formation of papules at the periphery of previously healed ulcers.19 Chronicity has been linked to numerous factors, including increased arginase activity in polymorphonuclear leukocytes.20 Diffuse CL, which is caused by L aethiopica, L Mexicana, or L amazonensis, presents as multiple nonulcerated papules and/or nodules involving most of the skin.5 The lesions contain abundant parasites and can result in significant facial changes, causing a leonine-like appearance similar to that seen in lepromatous leprosy. Mucosal lesions are common.21
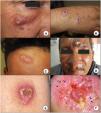
A–C, The presence of an erythematous papule or nodule exhibiting progressive growth and a tendency to ulcerate in exposed areas, such as the face or extremities, is the most characteristic presentation of cutaneous leishmaniasis. D, Some patients have multiple lesions, or atypical presentations such as the verrucous lesion in this patient with New World cutaneous leishmaniasis. Clinical (E) and dermoscopic (F) image of a cutaneous leishmaniasis lesion on the forearm showing central ulceration surrounded by an erythematous area with peripheral polymorphous and hairpin vessels (asterisks) and white-yellow teardrop-like structures (arrows)(polarized light, original magnification ×10).
Images courtesy of Dr. Morales Moya (2C), Dr. Galimberti (2D), and Dr Mayo Martínez (2E and F).
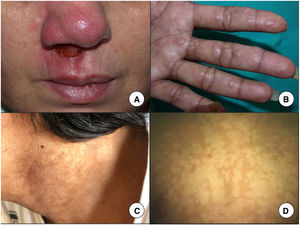
Mucosal involvement can coincide with cutaneous involvement or occur after the clearance of cutaneous lesions, sometimes even year later. The infection can spread through the bloodstream or lymphatic system. In endemic areas, mucosal involvement may be seen in up to 20% of patients.22 Most cases of MCL are caused by L braziliensis, but other species involved are L amazonensis, L guyanensis, and L panamensis. The nasal (Fig. 3A) and oral mucosa are the most frequently affected areas. Lesions in the oral cavity can spread to the oropharynx and larynx, and may affect cartilage and vocal cords.23 MCL lesions are ulcerated and can cause disfigurement. Treatment is essential to control infection, as the condition can be fatal.
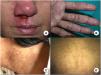
A, Mucocutaneous leishmaniasis manifests as a chronic ulcer often affecting the nasal mucosa. B, Firm cutaneous leishmaniasis papules on the palm of a patient coinfected with visceral leishmaniasis and human immunodeficiency virus. C, Hyperpigmented macules in the neck area. D, Hypopigmented macules as a manifestation of post-kala-azar dermal leishmaniasis in a 7-year-old boy. The diagnosis was established by molecular techniques, as the biopsy was negative.
In VL, infected macrophages spread through the reticuloendothelial system to the bone marrow, spleen, and liver. Clinical manifestations include fever, weight loss, hepatosplenomegaly, and lymph node enlargement. VL is mainly caused by L donovani in adults and L infantum or L chagasi in children and immunosuppressed individuals. Patients may also develop skin manifestations, which can be specific, such as papules (Fig. 3b), nodules, and ulcers, or nonspecific, such as purpura and hyperpigmentation (Fig. 3C). The presence of hyperpigmentation probably explains the origin of the term kala-azar, which means black fever in Hindu. Skin darkening has been described in 9.88% of patients with VL24 and was recently linked to an increased production of cortisol.25 Because hyperpigmented areas show amastigotes on histology, they could perhaps be considered a specific manifestation of leishmaniasis.26
Post Kala-Azar Dermal LeishmaniasisPost kala-azar dermal leishmaniasis (PKDL) occurs in patients with VL caused by L donovani or, on occasions, L infantum. It is particularly common in immunosuppressed patients27 and can appear up to 20 years after treatment.28 In patients infected with HIV, however, PKDL can coincide with, or at times precede, VL.29 It is characterized by hypopigmented macules (Fig. 3D), flesh-colored nodules and/or verrucous papules that mainly affect the face, although they can spread to the rest of the body.30 PKDL mainly occurs in east Africa and India, although cases are occasionally seen in Spain.31 The clinical presentation varies depending on geographic location and the host’s immune response. In Asia, 90% of cases appear as macules, while in Africa, papules are predominant.30 PKDL is more common—and severe—in immunosuppressed patients, who may exhibit atypical forms such as nodules not necessarily involving the face and a higher abundance of parasites in lesions.32 The main entity in the differential diagnosis is leprosy, which is characterized by a loss of sensation. PKDL must be treated systemically, although African forms can resolve spontaneously within a year.
HIV CoinfectionHIV infection has been linked to a 2000-fold increased risk of VL.33 This is because both infections share a immunopathogenic mechanism involving macrophages and dendritic cells that accelerates progression in both cases.34 HIV was responsible for the resurgence of VL in Europe during the 1990s that hit Spain, Portugal, Italy, and France particularly hard.33,35 Eighty percent of all cases of VL-HIV coinfection reported to the WHO during this period were from Spain.36 In addition, a retrospective analysis of all patients hospitalized for leishmaniasis in Spain between 1997 and 2008 found that 37% were HIV positive.37 Coinfection can result in atypical presentations of CL and VL,18,38,39 poor response to treatment, higher mortality rates, higher viral loads, and faster progression to AIDS. The introduction of highly active antiretroviral therapy in 1997 increased survival rates and also reduced the incidence and recurrence of leishmaniasis.33 It is important thus to rule out HIV in all patients with VL, even those living in endemic areas.3
Leishmaniasis and TNF InhibitorsTNF inhibitors are widely used to treat inflammatory diseases such as psoriasis and rheumatoid arthritis. TNF-α participates in cell-mediated immune response by activating CD4+ and CD8+ T cells. Together with other cytokines such as interleukin 12 and IFN-γ, it has a key role in early infection control. Parasite DNA has been detected in the blood of up to 58% of healthy individuals in the Mediterranean, an endemic area with high levels of exposure to Leishmania, but the actual numbers of people who develop the disease are low.40 It is therefore believed that most immunocompetent individuals are able to control infection before it manifests. Likewise, it has been suggested that TNF-α inhibitors could favor the reactivation of latent leishmaniasis,10 and also modify clinical presentation, the natural course of disease, and response to treatment. Based on the cases published to date, the best approach to dealing with reactivation of a latent infection would appear to be to treat the infection with systemic therapy and interrupt TNF inhibitor treatment until the infection has cleared10; the treatment should then be reintroduced with close follow-up. Etanercept and certolizumab appear to be associated with a lower risk of reactivation than adalimumab or infliximab.41
DiagnosisLeishmaniasis is diagnosed by demonstrating the presence of Leishmania amastigotes in clinical specimens using direct microscopic examination or molecular analysis based on nuclear or kinetoplast DNA amplification. Amastigotes are round and have a diameter of 1 to 4 μm and a characteristic rod-shaped structure known as a kinetoplast. The main sampling and diagnostic techniques are summarized in Table 2.42–45 Considering the limited sensitivity of some of the diagnostic techniques, it may be necessary to take several samples and/or use a combination of techniques to reach a diagnosis. In the case of biopsy specimens, for example, one part could be used for histology, another for touch imprint cytology, and another for culture. Diagnostic sensitivity in VL varies according to tissue type and can be as high as 90% for spleen tissue. Spleen aspiration is the procedure of choice for diagnosing VL, but it is associated with a high risk of intra-abdominal bleeding. Sensitivity rates ranging from 50% to 85% have been reported for bone marrow samples, and even lower rates have been described for lymph node and peripheral blood.46 In PKDL, diagnostic sensitivity using smears or biopsy specimens is largely dependent on the type of lesion analyzed, with rates of up to 100% for nodular lesions contrasting with very low rates for macular lesions,47 where more sensitive molecular methods are needed.48 More information on sampling methods and diagnostic techniques can be consulted in the guidelines of the Centers for Disease Control and Prevention49 and the Infectious Diseases Society of America.50
Sampling Methods and Diagnostic Techniques for Cutaneous and Mucocutaneous Leishmaniasis.
| Type of Lesion | Sampling Method | Diagnostic Techniques | Technique | Considerations | |
|---|---|---|---|---|---|
| Ulcer | Base | Swab. To collect DNA. Rub ulcer several times. | PCR | Real-time PCRSamples can be stored in high concentrations of ethanol or in paraffin, although paraffin-embedded samples yield lower sensitivity | Advantages: highest sensitivity and specificity, relatively fast, and allows identification of speciesDisadvantages: not always availableSensitivity: 100% |
| Touch print cytology. Remove the crust and take an imprint of the base of the ulcer on a slide. | Smear | SmearGiemsa staining and direct visualization using ×100 lens and oil immersionVisualization of amastigotes and their characteristic kinetoplast structure | Advantages: fast and cheap Disadvantages: does not allow identification of speciesSensitivity: 85% | ||
| Border | Fine-needle aspiration. Aspirate using a 1-mL syringe with a 20-25-G needle and 0.1 mL of sterile saline solution 0.9%. If no material is obtained, inject 0.05-0.1 mL of the saline solution and reaspirate. | Smear, culture, PCR | CultureSterile sampleMedium: Novy-MacNeal-Nicolle | Advantages: allows identification of species and definitive diagnosisDisadvantages: frequent contamination by skin flora and low sensitivitySensitivity: 40%Recent microculture techniques offer higher sensitivity (close to 100%), but do not allow identification of species | |
| Skin scrapings. Use a scalpel to obtain tissue scrapings from the upper dermis, via an incision or removal of necrotic tissue. Requires local anesthesia and bleeding control for better results. | Smear, culture, PCR | ||||
| Biopsy. Shave or punch. | Smear, culture, PCR, histology | HistologyHematoxylin-eosin | Advantages: can be used to rule out other possible causes, in particular, malignancyDisadvantages: invasive, does not allow identification of species, and has low sensitivitySensitivity: 60% | ||
| Nodule/plaque | Fine-needle aspiration | Smear, culture, PCR | |||
| Biopsy | Smear, culture, PCR, histology | ||||
Abbreviation: PCR: polymerase chain reaction.
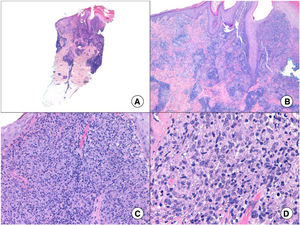
Histology can show nonspecific features such as ulceration, pseudoepitheliomatous hyperplasia, and a mixed inflammatory infiltrate, as well as specific features, such as amastigotes in dermal macrophages, seen in 50% to 70% of cases43 (Fig. 4). As lesions develop, there is an increase in the number of giant cells and a decrease in that of parasites. Other findings include tuberculoid granulomas,51 and in advanced stages, dermal fibrosis and abundant plasma cells.52 Four histologic patterns have been described for leishmaniasis: 1) abundant amastigotes (45%); 2) a mixture of macrophages, neutrophils, and plasma cells accompanied by necrosis (27.5%); 3) incipient granulomas with epithelioid cells, lymphocytes, and plasma cells (15%); and 4) fully formed epithelioid granulomas with Langhans-type giant cells.53 Histologic findings are similar in CL and MCL. A diffuse infiltrate of macrophages containing large numbers of amastigotes is seen in diffuse CL.52
A, Panoramic punch biopsy of an erythematous nodule on the forearm (hematoxylin-eosin, original magnification ×5). B, Dense superficial dermal inflammatory infiltrate and pseudoepitheliomatous hyperplasia (hematoxylin-eosin, original magnification ×10). C, Detail of inflammatory infiltrate composed mainly of macrophages, lymphocytes, and some epithelioid cells (hematoxylin-eosin, original magnification ×20). D, Characteristic amastigotes in infected macrophages (hematoxylin-eosin, original magnification ×40).
Dermoscopy is another useful diagnostic aid.54 The most frequently described dermoscopic structures are erythema (100%); vascular structures (90.6%), including polymorphous (40.2%), hairpin (39.4%), and arborizing vessels (38.6%); crusts (70.1%); and erosion/ulceration (44.1%). Less common but more characteristic structures are white-yellow teardrop-like structures (42.5%) and the white starburst pattern (8.6%)55 (Fig. 2F).
Other tests, which are less useful in the diagnosis of CL, are the Montenegro skin test and serological tests. The former consists of the intradermal injection of leishmanin, and its results are read and interpreted in a similar way to those of the tuberculin test. It is negative in diffuse CL, active VL, and PKDL,56 and cannot differentiate between current and past infection. It is mainly useful for epidemiological purposes.
Serological tests include the direct agglutination test, immunofluorescence, enzyme-linked immunoassay (ELISA), and Western blot analysis. These tests are highly sensitive in VL.57 Antibody titers, however, drop very slowly after cure and the test does not discriminate between active and past infection. Results can also be affected by cross-reactivity with other antibodies (e.g., Chagas disease).58 In addition, asymptomatic individuals in endemic areas often have antibodies, meaning that results have to be carefully interpreted according to the clinical context. High levels of anti-α-galactosyl antibodies have been detected using ELISA in CL caused by L tropica and L major.59 A number of rapid diagnostic tests, such as rK39, are also available. These have high sensitivity for VL,60 but they have the same limitations as other serological tests. More recent antigen detection tests include the latex agglutination test61 and ELISA62 with urine samples in VL and an immunochromatographic strip that tests for peroxidoxin antigen63 in CL.
Differential DiagnosisThe main entities that should be considered in the differential diagnosis are other infections, such as ecthyma, sporotrichosis, skin tuberculosis, furuncular myasis, subcutaneous mycosis, tertiary syphilis, and lepromatous leprosy; malignancies, such as squamous cell carcinoma, basal cell carcinoma, and lymphoma; and other skin disorders, such as persistent arthropod bite reactions, sarcoidosis, and granulomatosis with polyangitis.43
TreatmentMany cases of CL resolve spontaneously in under 2 years. Variations in resolution times are largely related to the species involved (Table 1), with infections caused by L braziliensis and L panamensis most likely to persist.64 Depending on this and other factors, such as anatomic location, infection severity, and the host’s immune response, CL can be classified as simple or complex. Simple infections can be treated conservatively or with local treatments, while complex infections require systemic therapies.50 The characteristics of simple and complex CL are summarized in Table 3. Multiple treatments exist for leishmaniasis, although the evidence supporting the options for CL is weak.65 The main treatments are shown in Table 4.66–97
Characteristics of Simple and Complex Cutaneous Leishmaniasis.
| Simple Cutaneous Leishmaniasis | Complex Cutaneous Leishmaniasis |
|---|---|
| Leishmania species associated with low likelihood of mucosal involvement | Leishmania species associated with high likelihood of mucosal involvement, particularly species from Leishmaniasis braziliensis complex |
| No notable mucosal involvement | Mucosal involvement, subcutaneous nodules, and/or significantly enlarged regional lymph nodes |
| Single or few lesions < 1 cm | ≥ 5 lesions measuring > 1 cm or single lesion measuring > 5 cm |
| Located in areas without significant cosmetic concerns and that are accessible for local treatment | Located on the face, ears, fingers or toes, and skin covering joints or genitals |
| Immunocompetent host | Immunosuppressed host (HIV, TNF inhibitors, etc.) |
| Lesions showing signs of spontaneous resolution at diagnosis | History of non-response to local treatment |
| No criteria for complex cutaneous leishmaniasis | Recurrent disease or diffuse presentation |
Abbreviations: HIV, human immunodeficiency virus; TNF, tumor necrosis factor.
Main Treatments for Cutaneous and Mucocutaneous Leishmaniasis.
| Treatment | Drug or Device | Indication | Dose, Adverse Effects and Other Considerations | Effectiveness97 | Level of Evidence1,a |
|---|---|---|---|---|---|
| Local TreatmentIntralesional | |||||
| Pentavalent antimonials | Sodium stibogluconate66–68 (Pentostam 100 mg/mL, foreign medication) | OWCLConsider for NWCL if no risk of mucosal involvement | Intradermal injection 0.2-0.5 mL (0.1 mL/cm2 in up to 5 sites/lesion every 3-7 d for up to 5 sessions)May require local anesthesiaMore effective when combined with cryotherapy70,71Intralesional administration does not usually cause adverse effects (except for local reactions), although QT interval prolongation has been reported in up to 25% of patients72 | 41%-98% (OWCL)77%-90% (NWCL)89%-91% (when combined with cryotherapy) | A (combined with cryotherapy, LCVM)B (NMCL) |
| Meglumine antimoniate69(Glucantime 1500 mg/5 mL) | |||||
| TopicalParomomycin73 | Paromomycin ointment74 (Leshcutan, paromomycin 15% + MBCL 12%, foreign medication) | Ulcerated lesions due to Leishmania spp. in OWCL and NWCL | Twice daily for 10-20 d | Similar to intralesional pentavalent antimonials in OWCL, and inferior in NWCL | A (OWCL) |
| Paromomycin 15% cream + gentamicin 0.5%75 (not commercially available) | Better responses in Leishmania major and Leishmania panamensis | B (NWCL) | |||
| Similar results for formulations with and without gentamicinCan cause intense inflammation | |||||
| Cryotherapy76 | Liquid nitrogen | Recent-onset CL lesionsResidual lesions after systemic treatmentConsider in pregnant and breastfeeding women and patients with contraindications for systemic treatment | 3 freeze-thaw cycles for 15-20 s, with halo of 1-2 mm; repeated every 3 wk until cureMore effective when combined with intralesional pentavalent antimonialsCan cause permanent hypopigmentation | 57%-75% (similar efficacy to intralesional pentavalent antimonials)89%-91% (if combined with intralesional pentavalent antimonials) | A (combined with IL APV, OWCL) |
| Thermoterapy77–79 | Application of superficial heat via radiofrequency (ThermoMed) | OWCL and NWCLConsider in pregnant and breastfeeding women and patients with contraindications for systemic treatment | 50 °C for 30 s applied to lesion and up to 1-2 mm of surrounding skin; 1-2 sessionsBased on in vitro studies showing that Leishmania parasites do not multiply at > 39 °C Requires local anesthesiaProduces second-degree burns | 48%-98% (OWCL)58%-90% (NWCL) | A (OWCL, NWCL) |
| PDT80 | Conventional PDT with ALA or MAL | OWCL, especially that caused by L major or Leishmania tropicaConsider in CL resistant to other treatments or in aesthetically sensitive areasNot recommended in CL due to Leishmania braziliensis complex or Leishmania donovani complex | 1-7 sessions a week; ≥ 3 sessions more effective than ≤2Mechanism of action believed to involve immune system response as does not directly kill the parasiteConventional PTD with ALA (6 sessions) seems to be at least as effective as cryotherapy (5 sessions) and produces better aesthetic results, although is more poorly toleratedLocal adverse effects (burning, reddening, edema, pain) | 96%-100% (conventional PDT)74%-82% (daylight PDT) | B (conventional PTD, OWCL) |
| Daylight PDT | |||||
| Intralesional PDT | |||||
| Systemic therapyOral | |||||
| [1,0]Azoles81 | Fluconazole | Off-label use for CL with lymphatic involvement and certain complex cases of CL; not indicated for cases due to L braziliensis | 200-400 mg/d for 42 dAdverse effects include gastrointestinal disturbances and hepatotoxicity | 44%-81% (OWCL)22% (NWCL) | A (OWCL, fluconazole 200 mg/d, 42 d, Lmajor) |
| Itraconazole | 200 mg/d for 42-56 d | ||||
| Miltefosine82–84 | Miltefosine (Impavido 50 mg capsules, foreign medication) | LC due to L braziliensis complex; can also be used in LC due to L major, L tropica, or Leishmania mexicana | 2.5 mg/kg/d (maximum 150 mg), administered orally in 3 doses for 28 dExpensiveTeratogenic and can cause gastrointestinal disturbances and hepatotoxicity | 81% (OWCL)53%-91% (NWCL) | B (NWCL)C (OWCL) |
| Parenteral | |||||
| Pentavalent antimonials85–88 | Sodium stibogluconate (Pentostam, 100 mg/mL) | [1,0]Complex OWCL and NWCL and MCL | 20 mg/kg/d in a single daily dose administered orally (preferred) or intramuscularly (very painful) for 10-30 dTreatment failures have increased due to resistance87Usually associated with pentoxifylline 400 mg 3 times a day for 10-20 d, or allopurinol 20 mg/kg for 30 days in recurrent LC due to L tropicaCan cause cardiotoxicity (e.g., arrhythmias, QT interval prolongation), pancreatitis, and hepatotoxicity | 41%-85% (OWCL)77%-90% (NWCL)30%-90% (MCL) | A (OWCM, MCL)C (recurrent CL) |
| Meglumine antimoniate(Glucantime 1500 mg/5 mL) | |||||
| Amphotericin B89–93 | Liposomal (AmBisome 50 mg), treatment of choice due to its lower toxicity compared with conventional options | Off-label use for complex CL and MCL | 3 mg/kg/d IV for 7 d (days 1-5, 14, and 21) up to a total dose of 21 mg/kg.Optimal dose for LC not well-defined; treatment regimens are based on the doses used for VL; higher doses and longer treatments are usually required in immunosuppressed patientsApproval of topical formulation of amphotericin 3% with similar efficacy to intralesional pentavalent antimonials in LC92Can cause nephrotoxicity, lower back pain, hypokalemia, chills | 84%-100% (OWCL, NWCL)78%-100% (MCL) | C (NWCL, MCL) |
| Pentamidine94–96 | Pentamidine isethionate 300 mg (Pentacarinat) | Alternative treatment for NWCL; good responses in Leishmania guyanensis | 4 mg/kg/d, intramuscular on alternating days for a weekCan also be administered intravenously (more effective).Intralesional administration recently approved; similar efficacy to pentavalent antimonialsCan cause severe toxicity: pancreatitis, QT interval prolongation, hyperpotassemia | 58%-95% (NWCL)91% (MCL, but 25% recurrence rate) | C (NWCL, MCL) |
Abbreviations: ALA, aminolevulinic acid; IL, intralesional; MAL, methyl aminolevulinate; MBCL, methyl benzethonium chloride; MCL, mucocutaneous leishmaniasis; NWCL, New World cutaneous leishmaniasis; OWCL, Old World cutaneous leishmaniasis; PDT, photodynamic therapy; VL, visceral leishmaniasis.
After weighing up the risks and benefits, a watch-and-wait approach can be taken in patients who meet the criteria for simple CL. Local treatment can be used for lesions that do not resolve spontaneously or when the goal is to achieve a faster cure or reduce the risk of scarring. The most widely accepted local treatments are intralesional pentavalent antimonials66–69 and cryotherapy.76 The combination of both produces better outcomes.70,71 Topical paromomycin is also used to treat CL, particularly in the New World.73–75
There have been reports of good responses to other local treatments,98 such as carbon dioxide laser therapy,99 which has shown efficacy rates of 93% and very few adverse effects (hyperpigmentation, persistent erythema, and hypertrophic scarring); photodynamic therapy80; and imiquimod.100
Systemic therapies are recommended for patients who meet any of the criteria for complex CL. Liposomal amphotericin B is highly effective but carries a risk of nephrotoxicity.89–93 The traditional use of systemic pentavalent antimonials85–88 to treat complex CL and MCL has led to resistance in certain areas, limiting their use.87 Other options include azoles,81 miltefosine,82–84 and pentamidine.94–96
A clinical trial comparing twice-daily chloroquine 250 mg and once-daily doxycycline 200 mg for 3 months reported an efficacy of 100% and 92%, respectively.101
Annual check-ups are recommended in patients with CL due to L braziliensis to enable early detection of progression to MCL. Signs of MCL include persistent nasal secretion or bleeding.
PKDL from Africa is not normally treated as the vast majority of cases (85%) resolve spontaneously within a year. In India, however, systemic treatment with miltefosine or amphotericin B is generally needed.1
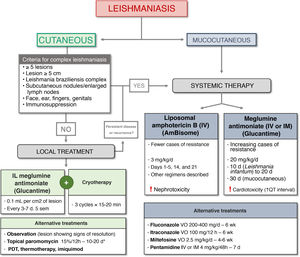
We have drawn up an algorithm for the management of CL and MCL based on current guidelines and expert recommendations (Fig. 5).
Prevention and ControlProphylactic vaccines for leishmaniasis in humans are not yet available. The fact that most patients who recover from leishmaniasis do not become reinfected facilitates vaccine research efforts.102 One of the main control strategies for CL and VL, apart from vector control, involves early detection and treatment, as infected patients are reservoirs. The WHO’s target for CL in the eastern Mediterranean region was to detect 70% of all cases and to treat at least 90% by 2020, an ambitious target considering the few treatment options available, the suboptimal diagnostic tools, and the low levels of awareness among the scientific community, particularly in relation to CL, which is not classified as an infection control priority.
ConclusionsCL has become a relatively common condition in our setting due to international travel and migration. Its management, however, can be complicated due to low indices of suspicion among clinicians, the low sensitivity of some diagnostic tests, poor access to molecular testing, limited treatment options, and adverse treatment effects. HIV coinfection and use of TNF inhibitors are associated with atypical presentations and the need for systemic treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Abadías-Granado I, Diago A, Cerro PA Palma-Ruiz AM, Gilaberte Y. Leishmaniasis cutánea y mucocutánea. ACTAS Dermo-Sifiliogr. 2021;112:601–618.