Pseudomonas aeruginosa is a gram-negative opportunistic bacillus of great relevance in dermatology due to its ability to cause a wide spectrum of skin infections. These infections can be characterized by a greenish discoloration and a distinctive odor and can range from mild signs, such as folliculitis, green nail syndrome, hot hand–foot syndrome, and uncomplicated acute otitis externa, which generally respond to conservative therapy and topical antibiotics, to deeper and potentially severe infections, such as subcutaneous nodules, malignant otitis externa, ecthyma gangrenosum, and necrotizing infections, which require a multidisciplinary approach, systemic antibiotics and surgical procedures. The growing antimicrobial resistance of P. aeruginosa poses a significant therapeutic challenge. This article provides a literature review focused on the various presentations and clinical signs of this pathogen, its resistance mechanisms, and the different therapeutic options.
Pseudomonas aeruginosa is a gram-negative, strictly aerobic, flagellated bacillus.1 It was first described in 1882 by Gessard,1 and produces two pigments: (1) pyocyanin, responsible for the greenish coloration of lesions and (2) pyoverdine, which produces a green fluorescence under Wood lamp examination.1 Its natural reservoir includes soil and plants, particularly in humid environments and aquatic settings.2P. aeruginosa infections are generally opportunistic but may also affect immunocompetent individuals.3 They exhibit a broad clinical spectrum, ranging from localized, self-limited conditions to potentially life-threatening systemic infections. P. aeruginosa possesses multiple virulence factors and antimicrobial resistance mechanisms4,5 (Table 1). Below, we review the various clinical presentations of P. aeruginosa infections with cutaneous involvement, including their clinical features, diagnosis, and treatment.
Microbiological characteristics of Pseudomonas aeruginosa.
| Flagellated gram-negative bacillus.Obligate aerobe.Reservoir: soil and plants, humid environments, and aquatic settings.Pigments: pyocyanin and pyoverdine.Virulence factors:• Thermal tolerance (up to 42°C).• Adhesion and colonization.• Biofilm formation.• Exopolysaccharides (lipopolysaccharide [LPS], Pel, Psl, alginate).• Secretion of toxins (ExoA, ExoS, ExoT, ExoU, ExoY), elastases (LasA and LasB), pigments, proteases (AprA), lipases (LipC), phospholipase C, esterase A, rhamnolipids, catalases (KatA, KatB, and KatE), reductases (alkyl hydroperoxide reductase), and superoxide dismutase (SOD).• Siderophores (pyoverdine, pyochelin).• Bacterial cell-to-cell communication (quorum sensing).• Secretion systems (T1SS, T2SS, T3SS, T4SS, T5SS, and T6SS).• Outer membrane vesicles (OMVs).Antibiotic resistance factors:• Quorum sensing.• Horizontal gene transfer (acquisition of resistance genes).• Production and integration of β-lactamases and carbapenemases.• Efflux pumps.• Target modification.• Membrane impermeability (specific and nonspecific porins).• Production of antibiotic-modifying enzymes.• Biofilm formation. |
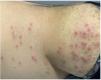
P. aeruginosa folliculitis is commonly associated with exposure to swimming pools, saunas, and hot tubs. Approximately 60% of pools and hot tubs may harbor this bacterium.6 Clinically, it is characterized by erythematous follicular papules and pustules that develop between 8h and 5 days (mean, 48h) after exposure to contaminated water7 (Fig. 1). It is often associated with pruritus and variable pain. Lesions typically appear in areas covered by swimwear, sparing the face and neck, as well as other occluded and apocrine-rich regions.1 This condition is generally self-limited, with spontaneous resolution within 7–14 days.3,8 In prolonged cases, cultures may be obtained, and treatment may include 2% acetic acid baths or topical therapies such as polymyxin B, tobramycin, gentamicin 0.1%, neomycin, benzoyl peroxide 5%–10%, chlorhexidine 0.5%–1%, or similar agents. In generalized or recurrent cases, oral fluoroquinolones such as ciprofloxacin may be prescribed.3,7,9
Superficial and generally mild cutaneous infections caused by Pseudomonas aeruginosa.
| Condition | Clinical signs | Management |
|---|---|---|
| Folliculitis | Perifollicular erythematous papules or pustules, often centered by a pustule, located in swimsuit-covered areas (upper trunk, skin folds, and buttocks). Typically appear 12–48h after exposure to contaminated water. Other symptoms include pruritus, variable pain, fever, and nausea. Usually resolve within 7–14 days. | Conservative or expectant management in most cases. 2% acetic acid baths. Topical antibiotics (polymyxin B, tobramycin, gentamicin 0.1%, neomycin), benzoyl peroxide 5%–10%, chlorhexidine 0.5%–1%, or similar agents.Oral ciprofloxacin 500mg every 12h for 7 days in selected cases. |
| Green nail syndrome | Classic triad of green discoloration of the nail plate (chloronychia), chronic proximal paronychia, and distal–lateral onycholysis.History of nail trauma or injury. | Avoid predisposing factors (moisture). Nail trimming.Topical agents: acetic acid, tobramycin, gentamicin 0.3%, sodium hypochlorite 2% solution twice daily for 1–4 months.Oral ciprofloxacin 500mg every 12h for 10 days. |
| Green foot syndrome (interdigital intertrigo) | Greenish discoloration of lesions, sweet “grape juice-like” odor, macerated or moth-eaten-appearing borders. Primarily affects interdigital spaces of hands and feet and skin folds. | Avoid predisposing factors (moisture). Debridement of macerated skin.Systemic antipseudomonal antibiotics according to antibiogram (usually ciprofloxacin 500mg every 12h for 10–14 days). Topical or systemic antifungal therapy if required. |
| Hot hands–feet syndrome | Painful nodules 1–2cm in diameter with red or purpuric erythema. Located on the palms and/or soles.Other symptoms include fever, malaise, abdominal pain, and nausea. | Conservative or expectant management.Symptomatic treatment (paracetamol, nonsteroidal anti-inflammatory drugs).In severe cases or immunocompromised patients: topical or systemic antipseudomonal antibiotics (fluoroquinolones, aminoglycosides, β-lactams such as ceftazidime or cefepime). |
| Acute external otitis | Otalgia, swelling of the external auditory canal, and local erythema, sometimes associated with maceration and greenish purulent discharge. | Removal of debris.Symptomatic treatment (paracetamol, nonsteroidal anti-inflammatory drugs).Otic drops: tobramycin, ofloxacin, ciprofloxacin 0.3%, ciprofloxacin 0.3% plus dexamethasone 0.1%, ciprofloxacin 0.2% plus hydrocortisone 1%, polymyxin B plus neomycin plus gramicidin, among others. Systemic antibiotics in selected cases. |
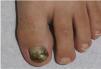
Green nail syndrome is a superficial nail infection characterized by green or blue-green discoloration of the nail plate10 (Fig. 2). Chronic proximal paronychia and distal-lateral onycholysis may also be present.10 Predisposing factors include frequent and prolonged water exposure, excessive detergent use, nail trauma, fungal coinfection, immunosuppression, nail psoriasis, and diabetes mellitus, among others.10 Diagnosis is clinical and confirmed by culture. Coinfection with dermatophytes is common, and fungal infection may facilitate P. aeruginosa colonization and overgrowth. Additionally, due to its fungistatic and/or fungicidal properties, P. aeruginosa may hinder fungal isolation in cultures.10,11 Management includes avoiding predisposing factors (moisture), trimming the nail, and topical application of 2% sodium hypochlorite, acetic acid, tobramycin, or gentamicin to the nail plate and cuticles for 1–4 months.3,12 Oral ciprofloxacin 500mg every 12h for 10 days may also be used.10,11
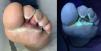
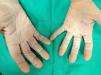
Hand and foot infectionsSuperficial cutaneous infections include green foot syndrome (Fig. 3), intertrigo, ulcers, and hot foot syndrome, among others.13 These conditions are characterized by greenish discoloration, a sweet “grape juice-like” odor, and macerated or moth-eaten-appearing borders.3,14 They primarily affect moist areas such as interdigital spaces of the hands (Fig. 4) and feet, as well as intertriginous regions. Coinfection with dermatophytes and other bacteria is common.3,15,16 Diagnosis is based on microbiologic culture. Management includes debridement of macerated skin and the use of systemic antipseudomonal antibiotics combined with topical treatments.17,18
Hot hand–foot syndrome is characterized by painful erythematous or purpuric nodules measuring 1–2cm on the palms and/or soles.19 It primarily affects children and may be accompanied by fever, malaise, and nausea.19,20 Histopathologic examination reveals a perivascular and perieccrine neutrophilic infiltrate extending into the subcutaneous tissue, with microabscess formation.19 Diagnosis is clinical, with exclusion of viral infections and eccrine hidradenitis, which is distinguished by neutrophilic infiltration of eccrine sweat glands and is typically triggered by cold exposure and mechanical stress.21 This condition is self-limited, resolving within 7–14 days. In highly symptomatic cases or in immunocompromised patients, anti-inflammatory agents and topical or systemic antipseudomonal antibiotics may be indicated.12,20,21
Acute uncomplicated otitis externa (“swimmer's ear”)Infection of the external auditory canal (EAC) caused by P. aeruginosa is relatively common in both children and adults. A recent study of patients with otitis externa and media found that P. aeruginosa was the second most frequent causative agent (24.4%).22 Otitis externa (OE) is characterized by otalgia, local erythema, and swelling of the EAC, sometimes accompanied by greenish purulent discharge.12 Diagnosis is clinical and confirmed by microbiologic culture. Treatment includes removal of debris and topical antibiotics that preferably also cover Staphylococcus aureus, such as tobramycin, ofloxacin, or ciprofloxacin combined with dexamethasone. Systemic antibiotics may be required in selected cases.3,23
Deep/severe infections caused by P. aeruginosa (Table 3)Malignant otitis externaMalignant otitis externa is a deep infection of the EAC that may lead to osteomyelitis, mastoiditis, facial nerve palsy, sepsis, sigmoid sinus thrombosis, and death. Major risk factors include immunosuppression, HIV infection, diabetes mellitus, and advanced age.24 It presents as a rapidly progressive condition with severe otalgia, persistent otorrhea, auricular swelling, lymphadenopathy, and granulation tissue formation.25 Diagnosis is based on imaging studies and positive cultures.26 Management is multidisciplinary and requires hospitalization and intravenous empirical antibiotics.27 Surgical debridement is indicated in cases of necrosis.12
Deep and potentially severe cutaneous infections caused by Pseudomonas aeruginosa.
| Condition | Clinical signs | Management |
|---|---|---|
| Malignant external otitis | Symptoms of acute external otitis.May involve intracranial structures, leading to osteomyelitis, facial nerve palsy, mastoiditis, sepsis, sigmoid sinus thrombosis, and death. | Hospitalization and multidisciplinary management.IV antibiotics (piperacillin–tazobactam, cefepime, ceftazidime, meropenem) with subsequent adjustment based on antibiogram; possible combination with an aminoglycoside (gentamicin, tobramycin) for 6 weeks to 6 months.Surgical debridement and other surgical procedures as needed. |
| Perichondritis | Inflammation, edema, erythema, and pain of the auricle.May present with abscess formation with or without underlying necrosis.History of local trauma, piercings, burns, surgery, or other entry points. | Hospitalization and multidisciplinary management.Systemic antibiotics: oral (ciprofloxacin) or IV (e.g., piperacillin–tazobactam, cefepime).Symptomatic treatment (paracetamol, nonsteroidal anti-inflammatory drugs).Surgical debridement and other surgical procedures as needed. |
| Subcutaneous nodules | Painful single or multiple subcutaneous nodules. Located anywhere on the body except palms and soles. Systemic symptoms in the context of bacteremia. More common in immunocompromised patients. | Hospitalization and multidisciplinary management.IV combination antipseudomonal antibiotics: aminoglycoside (amikacin, gentamicin, tobramycin) plus antipseudomonal penicillin (piperacillin–tazobactam, cefepime, ceftazidime, meropenem), followed by adjustment based on antibiogram.Surgical debridement and other surgical procedures as needed. |
| Ecthyma gangrenosum | Erythematous macules in the anogenital region or extremities progressing to vesicles and painful necrotic ulcers with surrounding erythema.Systemic symptoms such as fever, hypotension, and altered mental status. | Hospitalization and multidisciplinary management.IV combination antipseudomonal antibiotics: aminoglycoside (amikacin, gentamicin, tobramycin) plus antipseudomonal penicillin (piperacillin–tazobactam, cefepime, ceftazidime, meropenem), followed by adjustment based on antibiogram.Surgical debridement and other surgical procedures as needed. |
| Necrotizing infections | Edema, necrotic areas, severe pain.Systemic symptoms including fever, hypotension, and altered mental status. | Hospitalization and multidisciplinary management. Intravenous combination antipseudomonal antibiotics with adjustment according to antibiogram.Emergency surgical debridement and other surgical procedures as needed. |
Perichondritis is a rare complication involving the auricular cartilage. It presents with edema, erythema, and pain of the auricle and may progress to localized abscess formation with risk of necrosis. Predisposing factors include local trauma, piercings, burns, surgical procedures, and immunosuppression.28 Diagnosis is clinical, and management includes systemic antibiotics and surgical debridement when necessary.29
Subcutaneous nodulesThese lesions are usually due to P. aeruginosa bacteremia, particularly in immunocompromised patients,30,31 although they may also occur in immunocompetent individuals.32,33 Diagnosis is confirmed by biopsy showing a neutrophilic infiltrate with lobular panniculitis32 and by microbiologic cultures of subcutaneous tissue. Treatment requires systemic antipseudomonal antibiotics.
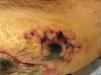
Ecthyma gangrenosumEcthyma gangrenosum (EG) is a severe infection classically described in immunosuppressed patients with bacteremia.34 Neutropenia is the main risk and prognostic factor,35 followed by lymphoproliferative disorders, malnutrition, diabetes mellitus, and extensive burns.36 Up to 74% of EG cases are caused by P. aeruginosa.37 A recent review showed that only 59% of patients presented with sepsis and that the classic triad of P. aeruginosa infection, sepsis, and immunosuppression was observed in only 19% of cases.37 In children, a recent retrospective study (n=17) found that most affected patients were immunosuppressed (14/17), with acute lymphoblastic leukemia being the most common cause; P. aeruginosa was isolated in 55%.38 EG initially presents as asymptomatic erythematous macules, most commonly in the anogenital region or extremities, which rapidly progress to painful vesicles and necrotic ulcers39 (Fig. 5). The most frequently affected areas are the gluteal/perineal region (57%), extremities (30%), trunk (6%), and face (6%).39 It is classically associated with fever, hypotension, and altered mental status.35,37 Histologically, a necrotizing hemorrhagic vasculitis with gram-negative bacilli in the medial and adventitial layers of deep vessels is observed.3 Diagnosis is based on clinical findings, local cultures, and blood cultures.3 Prompt treatment is essential, as mortality ranges from 10% to 70%.38 Some authors recommend combination IV antibiotic therapy with aminoglycosides (amikacin, gentamicin, tobramycin) and antipseudomonal penicillins (piperacillin–tazobactam, cefepime, ceftazidime, meropenem), along with surgical intervention when indicated.39,40
Superinfection of ulcers or burnsP. aeruginosa is one of the main etiologic agents of superinfection in ulcers and burns.41,42 Antibiotic multidrug resistance has increased in recent years.43 Major risk factors include prolonged hospitalization, use of broad-spectrum antibiotics, prior P. aeruginosa infections within the unit, and extensive burn surface area.44 Diagnosis relies on high clinical suspicion, with greenish or yellowish discoloration serving as suggestive clues.12 Management includes debridement of necrotic tissue and systemic and/or topical antipseudomonal antibiotics.45,46
Necrotizing soft tissue infectionsNecrotizing infections caused by P. aeruginosa are rare but associated with high mortality rates (≈30%) and may occur as part of polymicrobial infections.46 Risk factors include alcoholism, immunosuppression, and diabetes mellitus, among others.12 Clinically, they resemble other necrotizing infections, with necrotic areas, severe pain, and systemic symptoms. Management must be multidisciplinary and includes prompt surgical debridement and broad-spectrum combination intravenous antibiotic therapy.3,12
P. aeruginosa and antimicrobial resistanceThe management of severe cutaneous infections caused by P. aeruginosa is challenging. Fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin) are among the most widely used agents; however, resistant strains have increased in recent years.47 Multidrug-resistant P. aeruginosa strains are estimated to have increased by 15%–30% in Europe, North America, and South America.5 A 2019 review identified P. aeruginosa as one of the 6 pathogens responsible for deaths due to multidrug-resistant bacteria.48 A study evaluating P. aeruginosa in swimming pools and hot tubs found that 21% of samples contained the pathogen, and 96% were multidrug resistant to relevant antipseudomonal agents, including aztreonam (22%), gentamicin (9%), and imipenem (26%), with intermediate resistance to amikacin (9%), meropenem (4%), and tobramycin (9%). Resistance was also detected to ceftriaxone (4%), ticarcillin–clavulanic acid (4%), and trimethoprim–sulfamethoxazole (13%).6 A recent study from the global ATLAS (Antimicrobial Testing Leadership and Surveillance) program showed that carbapenem resistance between 2018 and 2022 ranged from 15% to 33%.49
Antimicrobial resistance has led to increasing use of combination antibiotic regimens and the search for new antimicrobial agents, with limited success. A recent review recommends ceftolozane–tazobactam and ceftazidime–avibactam for P. aeruginosa infections with limited therapeutic options.50 Other emerging options under investigation include cefiderocol, imipenem–cilastatin–relebactam, and meropenem–vaborbactam.50
DiscussionCutaneous infections caused by P. aeruginosa range from superficial conditions, which generally respond to conservative management and topical antibiotics, to deep and potentially severe infections, such as subcutaneous nodules, malignant otitis externa, ecthyma gangrenosum, and necrotizing infections, which require multidisciplinary management, systemic antibiotics, and surgical interventions. Traditionally, invasive or severe P. aeruginosa infections occurred primarily in immunocompromised individuals and were frequently associated with septic manifestations. However, recent studies have shown that these infections may also occur in a substantial proportion of immunocompetent patients.37
Dermatologists play a key role in the evaluation of a broad differential diagnosis that includes vascular, inflammatory, autoimmune, infectious, and neoplastic conditions, as well as in the appropriate performance of biopsies, both for histopathologic assessment and microbiologic culture. Wood lamp examination can be a useful, rapid, and inexpensive diagnostic tool for P. aeruginosa infections by detecting the characteristic green fluorescence.51 In severe cases, imaging studies, blood cultures, urine cultures, and laboratory tests – including complete blood count and C-reactive protein – should be obtained, as well as serum procalcitonin and lactate levels in patients with suspected sepsis.37 Therapeutic management should be guided by the clinical presentation, predisposing risk factors, and local community antimicrobial resistance patterns.12
Multidrug-resistant strains are associated with higher costs, prolonged lengths of stay, extended antimicrobial therapy, and increased complication rates.52 Because deep or severe P. aeruginosa infections, including ecthyma gangrenosum, may be clinically indistinguishable from infections caused by other pathogens, broad-spectrum antibacterial and antifungal coverage is recommended in neutropenic or septic patients, with subsequent adjustment based on local culture and blood culture results. In such cases, management in intensive care units is required.34,35
ConclusionsP. aeruginosa is responsible for a wide spectrum of cutaneous infections, ranging from mild and superficial conditions to severe and systemic disease. The increasing prevalence of antimicrobial resistance further complicates the management of these infections, underscoring the need for new therapeutic strategies and a multidisciplinary approach. A high index of clinical suspicion, prompt diagnosis with appropriate microbiologic confirmation (including antimicrobial susceptibility testing), and judicious use of antibiotics are essential.
Conflict of interestThe authors declare no conflict of interest.