Frontal fibrosing alopecia (FFA) is a scarring alopecia first described in 1994. Due to its increasing incidence rate in dermatology clinics, and the scarcity of approved specific treatments, the diagnosis and treatment of FFA can be an increasingly common challenge for dermatologists in their routine daily practice.
This FFA management consensus was produced via a Delphi methodology involving 22 dermatologists from the Spanish working group on trichology and onychology of the AEDV.
Because hair loss is irreversible, multimodal therapy with complementary mechanisms and routes should be used starting with oral dutasteride along with topical and/or intralesional agents; if inflammation persists, hydroxychloroquine should be added to the mix.
Frontal fibrosing alopecia (FFA) is a scarring alopecia that causes irreversible hair loss following a phase of variable inflammatory activity.1 Since the publication of the first case in 1994,2 the incidence rate of FFA has been steadily increasing in dermatology consultations.3 However, despite its growing incidence, the definitive causes of FFA remain unclear, although evidence suggests a genetic basis influenced by endocrine and environmental factors that trigger disease expression.4–7 From an epidemiological standpoint, FFA primarily affects postmenopausal women, although cases have also been reported in premenopausal women and, rarely, in men.8,9
Diagnosis of FFA is usually clinical, based on physical examination and trichoscopy, while biopsy is reserved for cases with diagnostic uncertainty.10 Several clinical patterns have been described: type 1 (linear pattern), type 2 (diffuse pattern)—associated with a poorer prognosis—and type 3 (double-line pattern); less common variants, such as the “tiara” pattern, have also been reported.11 Therapeutic management relies on 2 main pillars: topical and intralesional anti-inflammatory agents, and oral 5-alpha-reductase inhibitors.12,13 Additional treatments may include oral anti-inflammatory drugs such as hydroxychloroquine,14 or injectable therapies such as platelet-rich plasma (PRP).15 However, the absence of approved targeted therapies and the scarcity of randomized clinical trials remain major challenges for FFA management in dermatology practice.
Given the increasing number of new FFA diagnoses in our setting, the aim of this document is to provide an updated guideline on the diagnostic and therapeutic management of FFA, based on expert consensus and the available scientific evidence.
MethodsThis consensus was developed by members of the Spanish Group of Trichology and Onychology (GETO-AEDV) of the Spanish Academy of Dermatology and Venereology (AEDV) using a modified Delphi method. A scientific committee composed of eight GETO-AEDV members—each with over 5 years of experience in trichology—was formed. The project was presented to the GETO-AEDV assembly, and volunteers with prior scientific publications on FFA were invited to join the committee. A qualitative literature review was, then, conducted, including articles published in Spanish and/or English since 2014. Afterwards, a structured questionnaire was designed with 4 thematic blocks: general aspects, diagnosis, treatment, and special situations. All GETO-AEDV members were invited to participate as panelists. The questionnaire included 106 statements and was distributed online to 22 Spanish dermatologists, all GETO-AEDV members with extensive experience managing FFA. The study was conducted in full compliance with the principles outlined in the Declaration of Helsinki.
Two voting rounds were conducted in January and February 2025, where panelists rated each statement using a 9-point Likert scale (1=strongly disagree, 9=strongly agree). Responses were grouped as disagreement (1–3), neutral (4–6), and agreement (7–9). A statement was considered to have reached consensus if (1) the median score was within 1–3 or 7–9; (2) fewer than one-third of votes fell outside that range; (3) the interquartile range (IQR) was ≤3.
After the first round, the committee reviewed non-consensual items and revised wording where necessary. A second round of voting followed. At the end, the committee drafted the final consensus document, assigning each recommendation a Level of Evidence (LE) and Grade of Recommendation (GR) according to the Oxford Centre for Evidence-Based Medicine.16
ResultsIn the first round, a total of 95 items achieved consensus. After rewording 2 items, the second round yielded 8 additional consensual recommendations, totaling 103 of 106 statements (97%) (Supplementary data, Tables 1–4).
Recommendations for screening the most frequent comorbidities associated with FFA (LE 5; GR D).
| For all patients: |
| • Request thyrotropin (TSH) levels. |
| • If there are any changes to thyrotropin levels, expand the study by testing for antithyroid antibodies. |
| For patients with a personal or family history of autoimmune diseases: |
| • Request ANA levels and antithyroid antibodies. |
| For patients with a personal history of contact dermatitis, signs of lichen planus pigmentosus, or facial eczema: |
| • Consider performing a patch test for allergic contact dermatitis. |
FFA, frontal fibrosing alopecia; ANA, antinuclear antibodies; LE, level of evidence; GR, grade of recommendation.
Recommendations for the treatment of eyebrow involvement in patients with FFA.
| • A topical calcineurin inhibitor (tacrolimus or pimecrolimus) is recommended as first-line therapy for eyebrow involvement (LE 4; GR C).• A short initial course of topical corticosteroids may be prescribed, adjusting the dosage as needed to discontinue treatment based on the patient's progress (LE 4; GR C).• Oral minoxidil should be considered in cases of eyebrow involvement (LE 4; GR C).• Intralesional corticosteroid injections should be considered (LE 4; GR C).• Topical minoxidil 5% and/or prostaglandin analogs may be prescribed to enhance eyebrow hair growth and prevent progressive loss (LE 4; GR C). |
FFA, frontal fibrosing alopecia; LE, level of evidence; GR, grade of recommendation.
Recommendations for injectable treatment of FFA.
| Intralesional corticosteroid injections |
| • Intralesional corticosteroid injections should be added to topical therapy in patients with moderate or severe inflammation (LE 4; GR C). |
| • Triamcinolone acetonide is recommended due to its greater efficacy vs other intralesional corticosteroids, with a similar safety profile (LE 4; GR C). |
| • Inject≤0.1mL per injection at the dermal level (about 2mm deep) at inflammatory foci (LE 5; GR D). |
| • Local corticosteroid injections should be administered every 2–3 months within the first year (LE 5; GR D). |
| • Thereafter, they may be spaced every 6 months until inflammatory activity is controlled, while monitoring for atrophy (LE 5; GR D). |
| PRP injections |
| • Local PRP injections may have anti-inflammatory effects in FFA and may improve or prevent corticosteroid-induced and disease-related atrophy (LE 4; GR C). |
| • PRP and corticosteroid injections can be administered in the same session (LE 5; GR D). |
| • PRP may be injected into the dermis, or both the dermis and subcutaneous tissue (LE 5; GR D). |
| • If PRP therapy is prescribed, sessions should be scheduled every 3 months (LE 5; GR D). |
FFA, frontal fibrosing alopecia; PRP, platelet-rich plasma; LE, level of evidence; GR, grade of recommendation.
Criteria for hair transplantation in FFA (LE 5; GR D).
| The ideal candidate for hair transplantation in a patient with FFA should meet the following criteria:• Realistic expectations regarding treatment outcomes.• Awareness of the high risk of transplanted follicle loss in the medium to long term.• FFA stability for at least 12 months.• Absence of inflammatory signs on trichoscopy.• Small transplant areas (<1500 follicular units). |
FFA, frontal fibrosing alopecia; LE, level of evidence; GR, grade of recommendation.
Based on current evidence, panelists agreed that certain drugs—such as hormone replacement therapy, raloxifene, and oral contraceptives—are associated with a higher risk of developing FFA in genetically predisposed individuals. In addition, endocrine disruptors present in some cosmetic and sunscreen products may contribute to disease onset in these patients.
Panelists agreed that FFA diagnosis should rely on clinical and trichoscopic criteria. Trichoscopy is highly valuable, not only for initial diagnosis—recommended at the first consultation—but also for monitoring treatment response. Biopsy should be reserved for diagnostic uncertainty, such as atypical or early-stage presentations.
Given the possible association of FFA with other dermatologic and autoimmune diseases, recommendations for screening comorbidities are shown in Table 1.
Finally, the panel emphasized the importance of effective communication with patients regarding disease prognosis, to establish realistic expectations about treatment outcomes.
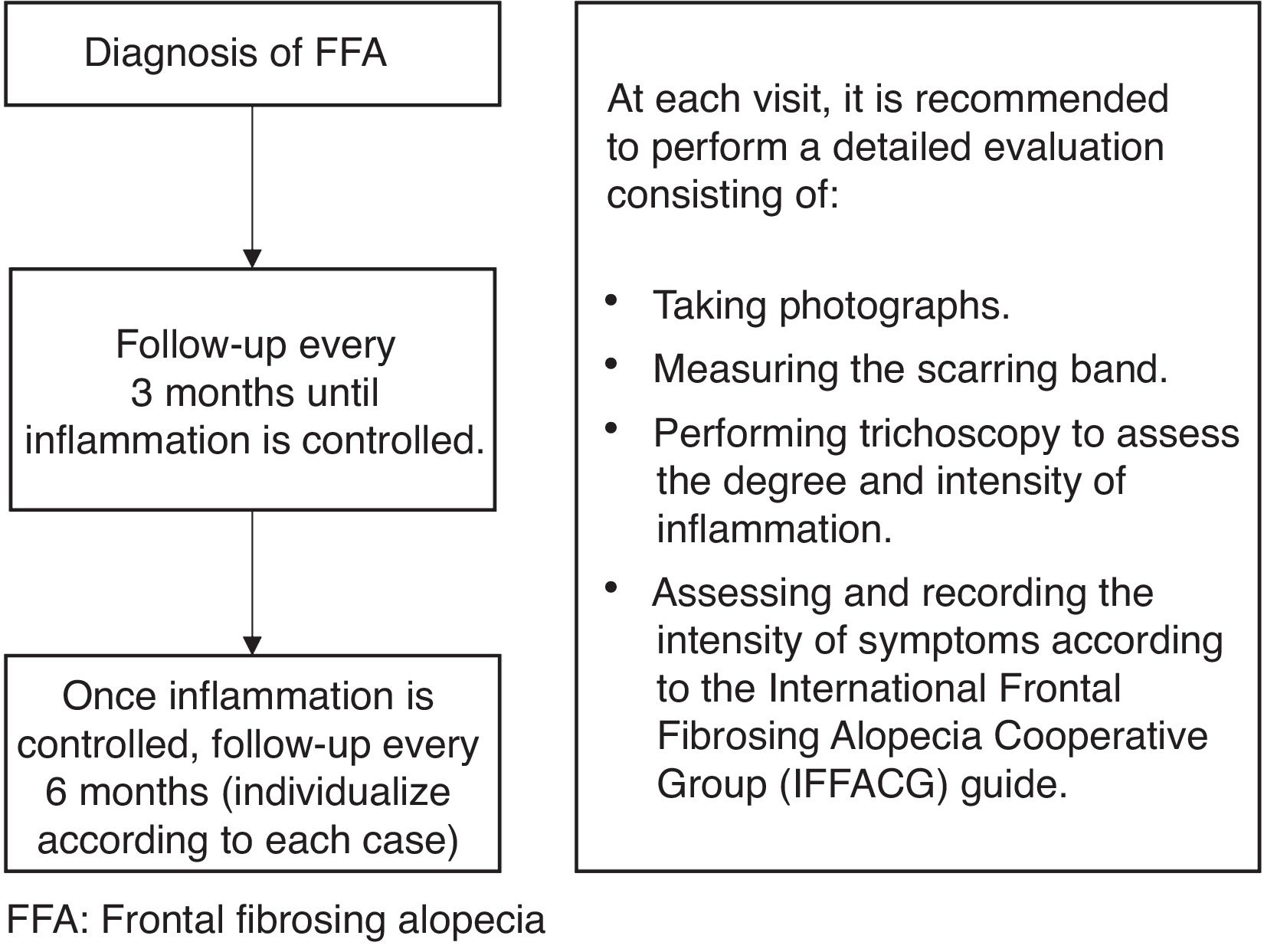
Recommendations on the therapeutic management of FFARegarding the therapeutic management of FFA, the panelists reached a consensus that, given the irreversible nature of hair loss in this condition, a combined treatment regimen should be used, employing drugs with different mechanisms of action and routes of administration to increase the likelihood of therapeutic success. After diagnosis, follow-up visits should be scheduled every 3–4 months until inflammatory activity is controlled. Once inflammation has been stabilized, patients should be seen every 6 months to monitor for new inflammatory flare-ups, although this frequency should be individualized based on the patient's condition, clinical findings, and associated symptoms. This follow-up scheme, along with specific recommendations for FFA monitoring at each visit, is shown in Fig. 1 and Supplementary Fig. 1 (Supplementary data).
Optimal medical therapy should be tailored according to the progression of alopecia and the degree of inflammation, always in agreement with the patient and considering their preferences to improve treatment adherence. Therapy should be maintained at least until stabilization of alopecia and resolution of inflammatory signs. The recommendations for considering discontinuation of medical treatment are presented in Supplementary Table 5 (Supplementary data).
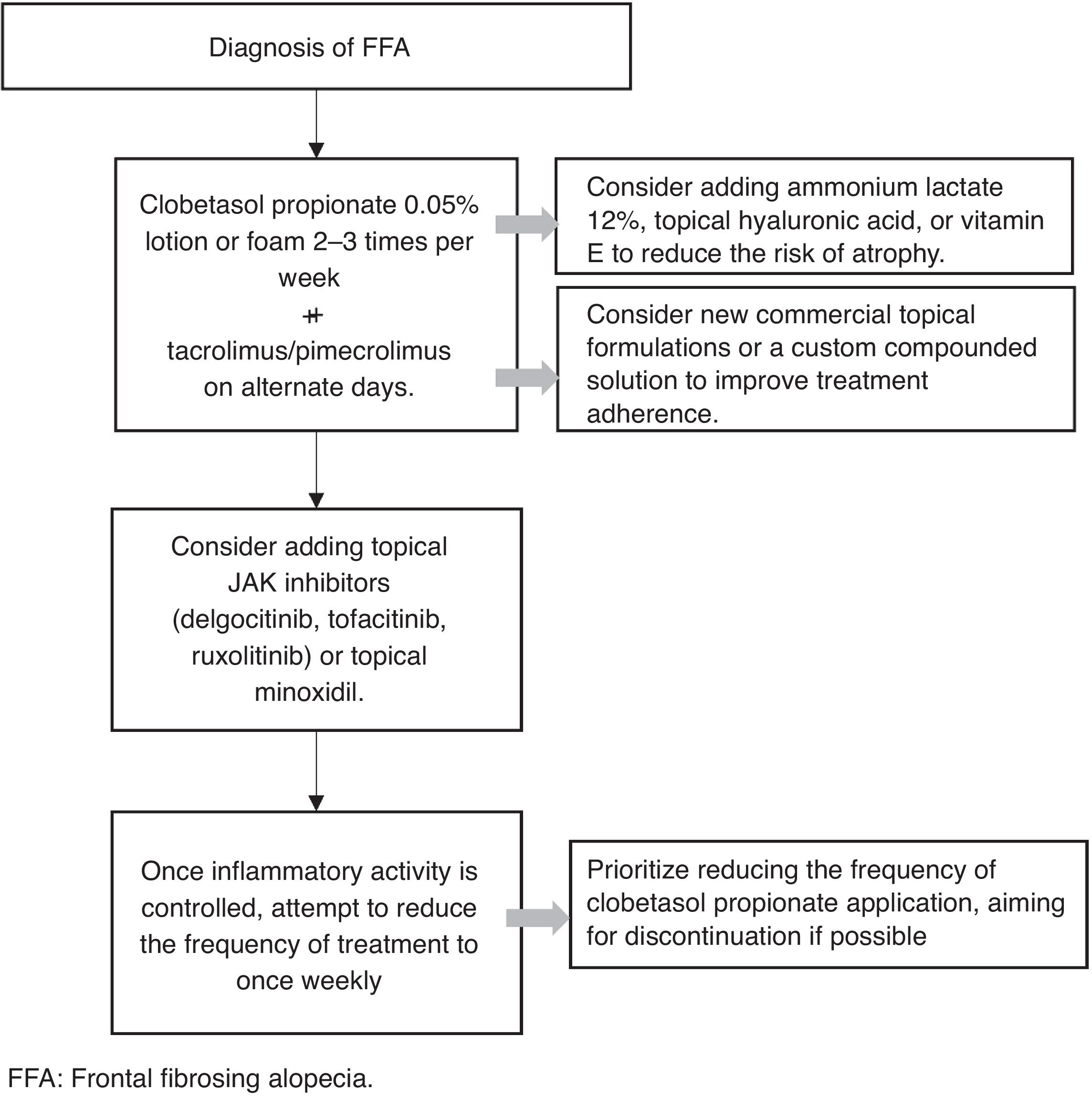
Topical treatmentThe panelists agreed that the first-line therapy for FFA is topical treatment with a very high-potency corticosteroid along with a calcineurin inhibitor to reduce the risk of skin atrophy associated with chronic topical corticosteroid use. The algorithm for topical treatment along the frontal hairline is summarized in Fig. 2.
Regarding the treatment of eyebrow involvement, the panelists’ recommendations are summarized in Table 2.
Injected treatmentIntralesional corticosteroid injections should be added to topical therapy in patients presenting with a moderate or severe degree of inflammation. While triamcinolone acetonide was recommended for its greater efficacy vs other intralesional corticosteroid options, there was no consensus on the maximum concentration of this drug. However, concentrations equal to or greater than 8mg/mL were discouraged for the treatment of both the frontal hairline and the eyebrows. Regarding other injectable treatments, local injections of platelet-rich plasma (PRP) may have an anti-inflammatory effect, improving or preventing both corticosteroid-induced atrophy and the disease-related atrophy per se. PRP injections may be administered in the same session as intralesional corticosteroid therapy. The recommendations for injectable treatment of FFA are shown in Table 3.
Oral treatmentOral 5-alpha-reductase inhibitors should be prescribed as first-line systemic therapy in combination with topical and/or intralesional agents, unless contraindicated or declined by the patient. Dutasteride 0.5mg/day was recommended for its greater potency vs finasteride, with a similar safety profile.
Regarding oral anti-inflammatory therapy, hydroxychloroquine at an initial dose of 4.5mg/kg/day is considered the treatment of choice in patients with FFA who show persistent inflammatory activity despite first-line topical and intralesional therapy, or in those presenting with poor prognostic factors at diagnosis. In exceptional cases, when hydroxychloroquine treatment fails, the addition of other anti-inflammatory agents such as cyclosporine, mycophenolate mofetil, or methotrexate may be considered. Similarly, in patients with rapidly progressive FFA, the panelists agreed on the possibility of prescribing a short course of oral corticosteroids.
Regarding other oral therapies, oral minoxidil may be used as an adjuvant treatment for FFA, and low-dose isotretinoin (20–35mg/week) may be prescribed in the presence of facial papules.
Reconstructive treatmentsConcerning hair transplantation, the panelists agreed that this is not a first-line technique for all FFA patients, due to the high risk of transplanted follicle loss in the mid-to-long term. Therefore, they emphasized the importance of careful patient selection, as well as clear communication regarding long-term outcomes and ensuring that patients have realistic expectations. The criteria for an ideal candidate for hair transplantation as a reconstructive option in FFA are listed in Table 4.
It was also agreed that microblading may be a good esthetic option for patients with partial eyebrow alopecia, as it carries fewer side effects than micropigmentation and provides a more natural result. To perform microblading, the patient should be under treatment and free of inflammatory signs. Patients should be informed that results may last a shorter time than in the general population (approximately 6 months vs 1 year) and that color changes (from brown to gray) may occur. Microblading, micropigmentation, and traditional micropigmentation are all compatible with other FFA treatments.
Other treatments and recommendationsConsensus recommendations regarding other treatments, including low-level laser therapy (LLLT) and excimer laser, are summarized in Table 5.
Recommendations for other treatments in the management of FFA.
| Low-level laser therapy (LLLT): |
| • LLLT may be useful as an adjuvant therapy to improve hair follicle thickness and growth and to reduce inflammatory signs (hyperkeratosis and perifollicular erythema) (LE 4; GR C). |
| • As an adjuvant treatment, LLLT should be used 3–7 times per week for 6 months, then 1–2 times per week thereafter (LE 5; GR D). |
| • Home-use devices are recommended for easier long-term application (LE 5; GR D). |
| Excimer laser therapy: |
| • Excimer laser may be considered as an adjuvant treatment in FFA due to its anti-inflammatory and immunomodulatory activity (LE 5; GR D). |
| • If prescribed, excimer laser should be applied in-clinic 2 to 3 times per week for an initial period of 3 months (LE 5; GR D). |
| • Treatment may be extended beyond 3 months if necessary (LE 5; GR D). |
| Cosmetic products: |
| • There is no evidence to support the use of current nutricosmetics for improving FFA (LE 5; GR D). |
| • Patients with FFA should avoid cosmetic products containing endocrine disruptors such as benzyl salicylate (LE 4; GR C). |
| • Sunscreens containing insoluble inorganic (mineral) filters are recommended, while those with soluble organic filters should be avoided (LE 4; GR C). |
FFA, frontal fibrosing alopecia; LLLT, low-level laser therapy; LE, level of evidence; GR, grade of recommendation.
Consensus recommendations for the treatment of FFA in special situations are summarized in Table 6.
Recommendations for the treatment of FFA in special situations (LE 5; GR D).
| Recommendations for treating FFA in men: |
| • Involvement of the beard and sideburns, body hair loss, association with lichen planus, or the presence of facial papules should raise suspicion of FFA in men. |
| • Treatment of FFA in the beard area should include a topical calcineurin inhibitor, limiting topical corticosteroids due to the risk of skin atrophy. |
| • Local corticosteroid injections may be used in the beard area with caution and on a case-by-case basis. |
| Recommendations for treating FFA in premenopausal women: |
| • In premenopausal women, reproductive plans and potential pregnancy should be carefully considered when selecting therapy. |
| • In premenopausal women desiring pregnancy, hydroxychloroquine may be prescribed as first-line systemic therapy along with topical clobetasol and a calcineurin inhibitor. |
| • Pregnancy should be avoided during dutasteride therapy and for 6 months after discontinuation. |
| • Pregnancy should be avoided during isotretinoin therapy and for 1 month after discontinuation. |
| • During pregnancy, if needed and after individual assessment of risks and benefits, topical clobetasol, local triamcinolone injections, and platelet-rich plasma may be continued. |
| • Topical calcineurin inhibitors and oral minoxidil may be initiated and maintained during breastfeeding. |
FFA, frontal fibrosing alopecia; LE, level of evidence; GR, grade of recommendation.
This consensus document provides practical recommendations for the diagnosis and therapeutic management of FFA in routine clinical practice, based on both available scientific evidence and expert opinion. Although FFA diagnosis can generally be made easily using clinical and trichoscopic criteria, there may be diagnostic uncertainty in certain cases—such as atypical presentations or male patients—where the involvement of a dermatopathologist specialized in trichology is essential.17,18 While endocrine disruptors have been involved in the pathogenesis of FFA, controversy surrounds the use of cosmetic products containing such agents in patients with FFA.4,19–21 Based on the available evidence, the panel recommended avoiding products containing these substances, such as organic soluble filters (in sunscreens) or benzyl salicylate (in other cosmetics).
Because of the rising incidence rate of FFA in dermatology practice, it is likely that most dermatologists will eventually face the therapeutic challenge of managing this condition.3 The lack of approved targeted therapies and the limited scientific evidence supporting current treatments were identified as major obstacles in this consensus.22 However, other aspects of management can also generate uncertainty—for example, determining the optimal timing for treatment withdrawal. Considering the risk of symptom recurrence,23 the experts agreed on criteria to guide treatment discontinuation, while leaving open the possibility of indefinite chronic therapy, provided it is discussed and agreed upon with the patient. As it happens with other trichologic diseases, the panel emphasized the importance of clear patient communication and accurate information sharing regarding disease prognosis, to foster realistic expectations about potential treatment outcomes.24,25
Regarding optimal medical therapy, and given the irreversible nature of hair loss in FFA, the consensus advocates for a combined therapeutic approach from diagnosis, including systemic therapy from the outset in patients with poor prognostic factors.12,13,23,26,27 As first-line therapy, unless contraindicated or declined, the combination of high-potency topical corticosteroids with a calcineurin inhibitor and oral dutasteride was recommended.28,29 Intralesional corticosteroid injections may be added in cases with moderate or severe inflammatory activity. Among other oral agents, hydroxychloroquine was positioned as the anti-inflammatory drug of choice in patients with severe inflammatory activity or poor prognostic indicators at diagnosis. In these cases, ophthalmologic monitoring is important due to the potential risk of retinopathy.30 While there is some evidence supporting the use of topical JAK inhibitors as adjuvant therapy, no clinical trials have yet evaluated the safety and efficacy profile of oral JAK inhibitors for FFA treatment.31,32 However, it is possible that these agents may become part of the therapeutic arsenal in the future.33
This study has several limitations, including the lack of randomized clinical trials evaluating FFA treatments, which reduces the level of evidence and strength of recommendations for most of the statements included in this consensus. Furthermore, the selection criteria used to include panelists—restricted to GETO-AEDV members—could have introduced a selection bias.
ConclusionsGiven the increasing incidence rate of FFA in dermatology practice and the scarcity of approved specific treatments, managing this condition is likely to become an increasingly common challenge for dermatologists. This national consensus document aims to serve as a guideline for the diagnosis and management of FFA in routine clinical practice.
Conflicts of interestDavid Saceda-Corralo declared to have received honoraria for participating in strategic advisory board meetings for Pfizer and as a speaker for Pfizer, Lilly, L’Oréal, and Cantabria Labs.
Andrea Combalia declared to have received honoraria as a speaker for Lilly, Pfizer, Isdin, and Cantabria Labs.
Cristina Pindado-Ortega declared to have received honoraria as a speaker for Novartis, Janssen, Lilly, ISDIN, UCB, Sanofi, Almirall, and Leo Pharma.
Sergio Vañó-Galván declared to have received honoraria for participating in strategic advisory boards for Pfizer, Lilly, and Cantabria Labs, and as a speaker for Pfizer, Lilly, L’Oréal, Pierre Fabre, and Cantabria Labs.
Pablo Fernández-Crehuet, Gloria Garnacho, María Librada Porriño-Bustamante, and Cristina Serranodeclare declared no conflicts of interest whatsoever.
The authors thank Dr. Bernadette Pfang and Dr. Pablo Rivas on behalf of Content EdNet for their assistance in manuscript preparation.
Ángel Aguado García, Diego Buendía Castaño, María Antonia Fernández Pugnaire, Blanca Ferrer Guillén, Pablo Fonda Pascual, Manuel Galán Gutiérrez, Rocío Gil Redondo, Alba Gómez Zubiaur, Elena González Guerra, Ramón Grimalt, Ángela Hermosa Gelbard, Maribel Iglesias Sancho, Juan Jiménez Cauhé, Alejandro Lobato Berezo, Paola Maldonado Cid, José María Mir Bonafé, Óscar Muñoz Moreno-Arrones, Daniel Ortega Quijano, Luis Puig Sanz, Ana Rita Rodrigues Barata, David Vega Díez, and Virginia Velasco Tamariz.