The complete regression of melanocytic tumors, confirmed by histology, has rarely been reported in the literature. It is very difficult to determine the malignant or benign nature of a regressed tumor, and on occasions, the only indication of malignancy is the subsequent development of metastasis.
Material and methodsWe performed a descriptive study of melanocytic nevi that had undergone complete, histologically confirmed regression prior to excision in the dermatology department of our hospital over a period of 3 years. We included only lesions in which dermoscopy performed prior to regression showed features that suggested benignity. We assessed various clinical, dermoscopic, histologic, and immunohistochemical features.
ResultsThe mean time to complete regression was 6.4 months. The main dermoscopic patterns observed were reticular and mixed reticular/globular. Unlike what is generally seen in melanomas, the main histologic finding was the presence of fine or lamellar fibrosis. In all cases, there was a predominance of CD8+ T cells.
ConclusionsThe clinical, dermoscopic, and histologic features of the melanocytic nevi studied suggest the existence of a highly characteristic form of tumor regression characterized by very rapid regression and the involvement of a cytotoxic mechanism.
La regresión completa de neoplasias melanocíticas, confirmada mediante estudio histológico, se ha descrito de forma excepcional en la literatura. En esos casos es muy complicado discernir si la lesión previa era maligna o benigna, y en ocasiones tan solo la presencia o ausencia de metástasis en el seguimiento posterior del paciente permite establecer dicha distinción.
MétodosSe realizó un análisis descriptivo que incluyó los nevos melanocíticos extirpados en nuestro Servicio de Dermatología en un período de tres años, en los que el estudio histológico demostró una regresión completa de los mismos. Se analizaron exclusivamente aquellas lesiones de las cuales disponíamos de un control dermatoscópico previo a la regresión completa de las mismas, y cuyas características dermatoscópicas sugerían que se trataba de lesiones benignas. Se valoraron diversos parámetros clínicos, dermatoscópicos, histológicos e inmunohistoquímicos.
ResultadosLa media del tiempo en que se produjo la regresión completa fue de 6,4 meses. Los patrones dermatoscópicos predominantes en los nevos con anterioridad a su involución fueron el reticular y la mezcla de reticular-globular. Histológicamente, el hallazgo más llamativo es la presencia de una fibrosis laminar o delicada, distinta a la que se aprecia habitualmente en melanomas. En todos los casos hubo un predominio de linfocitos T CD8.
ConclusionesLas características clínicas, dermatoscópicas e histológicas de las lesiones melanocíticas estudiadas sugieren que existe una forma muy particular de regresión de nevos melanocíticos que se caracteriza por la gran rapidez con que se produce la misma y que se originaría por un mecanismo citotóxico.
The spontaneous regression of tumors is among the most intriguing phenomena in medicine. This rare event has been described in nearly all types of cancer and occurs frequently in both benign and malignant melanocytic tumors in the skin, where depigmentation makes the process more visible.1–3 The mechanisms that have been implicated in regression include immune responses, apoptosis, inhibition of angiogenesis, terminal differentiation, and genomic instability.4–6
An immune mechanism seems to be the one that is most consistently associated with melanocytic tumor regression.2
Although 10% to 35% of melanomas regress partially, histologically confirmed complete regression of these tumors is very rare, with an estimated incidence of between 0.22% and 0.27%.7,8 To date, fewer than 50 cases have been documented, although underdiagnosis is likely.9–14 The largest melanoma series have found that the primary tumor has not been identified in 2.5% to 4% of metastatic melanomas, implying that these cases may correspond to tumors that have completely regressed after spreading.7,14–16
The complete regression of melanocytic nevi is seen far more often, however. The features of the halo nevus have traditionally been thought to typify the regressing lesion, although different patterns of involution have been identified since the introduction of digital dermoscopy.17,18
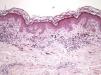
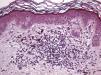
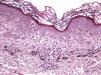
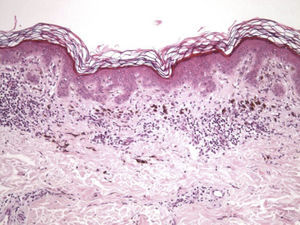
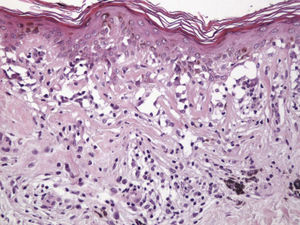
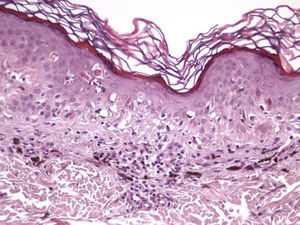
The essential feature of a melanocytic nevus undergoing spontaneous regression is the replacement of tumor cells by a fibrous stroma with varying degrees of inflammation and new blood vessel formation as well as varying numbers of melanophages (Fig. 1).7,19 From an exclusively histologic perspective, it is very difficult to determine the biology of a lesion after complete regression and in most cases only the later detection of metastasis on follow-up allows distinctions to be made.
This study aimed to analyze the clinical, dermoscopic, and histologic characteristics of a series of melanocytic nevi whose complete regression was confirmed histologically and to see whether it would be possible to identify clinical or histologic features that could distinguish them from completely regressed melanomas.
Materials and MethodsThis descriptive, observational, retrospective study was performed at Hospital Clínico Universitario de Valencia, Spain, over a 3-year period from January 1, 2007 to December 31, 2009. All melanocytic nevi removed by the hospital's dermatologists were included if complete regression was confirmed on histology. Regression was considered complete when histology showed complete absence of melanocytic proliferation in the epidermis or dermis, confirmed by Melan-A and S-100 immunohistochemical staining; other conditions were observation of a variable amount of infiltrate containing mononuclear cells and melanophages and the presence of blood cell proliferation. Excised lesions were examined only if we had evidence that the nevus had previously appeared benign during follow-up by digital dermoscopy. We also excluded lesions surrounded by a clinically evident whitish halo.
The 13 melanocytic nevi analyzed were found in patients who were examined in the digital dermoscopy unit of our department every 3 to 6 months because of the presence of multiple nevi or clinically atypical ones. Lesions were removed within 2 weeks of identifying any morphologic changes.
We then recorded the following information for analysis.
Patient and Lesion DataWe recorded patient age and sex and the location and size of the lesion as well as the time to complete regression.
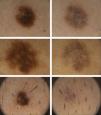
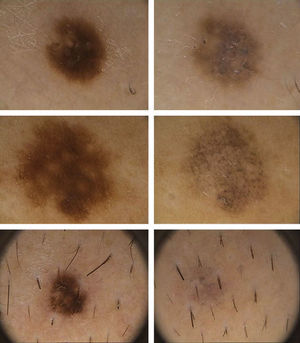
Dermoscopic DataThe dermoscopic observations assessed for each excised nevus were the predominant structural pattern identified before regression (globular, reticular, homogeneous, parallel furrow, or mixed) as well as the dermoscopic features of the lesion on regression (depigmentation and loss of structure; presence of a grayish-whitish veil, grayish pepper-like granules, or vascular structures).
These data were obtained by analyzing video recordings of the pigmented lesions made with a digital dermoscope (FotoFinder Systems GMGH).
Histologic DataWe recorded fibrosis intensity (none, slight, moderate, extensive) and type (compact, laminar, or mild-delicate), subepidermal fibrosis (present or absent), mononuclear inflammatory infiltrate (none, mild, moderate, or extensive), melanophages, (none, few, some, abundant), new blood vessel formation (none, slight, moderate, extensive), tumor cell apoptosis (none, slight, moderate, or extensive), and epidermal atrophy (present or absent).
Qualitative assessments were based on proportions of the regressed area occupied by each feature studied as follows: none present; mild-slight, present in up to a third of the area; moderate, present in up to two-thirds of the area; and extensive, involving two-thirds of the area or more.
Immunohistochemical FindingsWe performed immunostaining for CD4+ and CD8+ T cells as well as for Melan-A antibodies and S-100 protein. CD4+ and CD8+ T-cell immunoreactivity was assessed in terms of the percentage of the infiltrate occupied by the predominant cell type; when no inflammatory infiltrate was evident, this analysis was not performed.
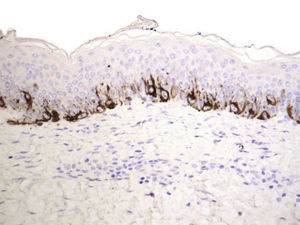
We used S-100 and Melan-A immunostaining to identify any residual nests of melanocytes or residual lentiginous hyperplasia.
All excised tissues were fixed in a neutral formaldehyde solution and embedded in paraffin for sectioning into 4-μm slices for histology and immunohistochemistry.
Histologic and immunohistochemical features were examined under a multiheaded microscope (Olympus).
Immunoperoxidase staining techniques were used.
ResultsClinical FindingsWe studied 13 lesions in which complete regression had been histologically demonstrated; 7 patients (53.8%) were men and 6 (46.2%) were women. The mean patient age was 36.3 years (range, 18-66 years).
Lesions were taken from the trunk in most cases (69.2%, 9 cases), mainly from the chest (38.4%, 5 cases) or back (30.8%, 4 cases). Of the remaining lesions, the locations were the hand (palm) or foot (sole) in 2 cases (15.3%); the arms in 1 case (7.6%); and the legs in 1 case (7.6%).
The mean diameter of the excised lesions was 0.5cm (range, 0.2-2cm).
The mean time to complete regression of the melanocytic nevi (months between the last dermoscopic examination and transformation of the nevus) was 6.4months (range, 3-11months).
Dermoscopic FindingsThe frequencies of morphologic features visible by dermoscopy are shown in Table 1.
Frequencies of Dermoscopic Features Observed.
| Structural Pattern | Percentage |
| Before regression | |
| Reticular | 30.7 |
| Mixed reticular-globular | 30.7 |
| Homogeneous | 15.3 |
| Parallel furrow | 15.3 |
| Globular | 7.6 |
| After complete regression | |
| Loss of structural pattern | 100 |
| Fibrosis (whitish) | 76.9 |
| Granules (pepper-like) | 53.8 |
| Vascular structures | 7.6 |
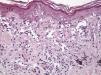
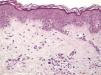
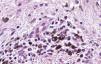
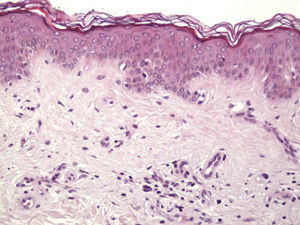
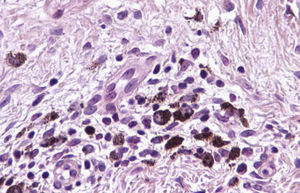
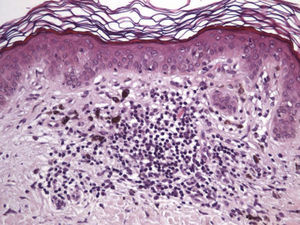
Histologic observations (Table 2) are exemplified in Figs. 2–6.
Frequencies of Histologic Features Observed.
| Structural Pattern | Percentage |
| Fibrosis | |
| None | 0 |
| Slight | 53.8 |
| Moderate | 46.1 |
| Extensive | 0 |
| Laminar | 38.4 |
| Delicate | 61.5 |
| Compact | 0 |
| Subepidermal location | 38.4 |
| Melanophages | |
| None | 0 |
| Few | 53.8 |
| Some | 23 |
| Abundant | 15.3 |
| Lymphocytic infiltrate | |
| None | 15.3 |
| Mild | 30.7 |
| Moderate | 53.8 |
| Extensive | 0 |
| Vascular proliferation | |
| None | 0 |
| Slight | 38.4 |
| Moderate | 46.1 |
| Extensive | 15.3 |
| Atrophy | 15.3 |
| Apoptosis | 30.7 |
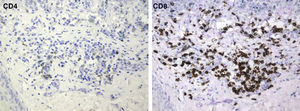
The mean percentage of CD4+ T-cell immunoreactivity was 23.1%; the mean percentage of CD8+ activity was 76.9%.
Residual lentiginous hyperplasia (Fig. 7) was observed by Melan-A staining in 15.38% of the lesions. No residual nests of melanocytes were detected.
The clinical, dermoscopic, histologic, and immunohistochemical findings are listed in Table 3.
Clinical, Dermoscopic, Histologic, and Immunohistochemical Features Observed in Each Lesion.
| Case | Age, y | Sex | Location | Diameter | Time to Regression | Dermoscopy | Fibrosis | Melanophages | Lymphocytic Infiltrate | Blood Vessels | Atrophy | Apoptosis | IHC |
| 1 | 61 | M | Chest | 0.2cm | 5 mo | Reticular, structureless, FB | ++DelicateSuperficial | + | + | ++ | No | No | 40% CD4+60% CD8+No LH |
| 2 | 26 | F | Leg | 0.2cm | 7 mo | Reticular, structureless, FB | +LaminarSuperficial | +++ | ++ | ++ | No | No | 10% CD4+90% CD8+LH |
| 3 | 26 | M | Back | 0.8cm | 5 mo | Reticular, structureless, P-L granules, FB | +DelicateNot superficial | ++ | + | ++ | No | +++ | 20% CD4+80% CD8+No LH |
| 4 | 21 | F | Back | 0.3cm | 7 mo | Reticular/globular, structureless, P-L granules, FB | +DelicateSuperficial | + | ++ | + | No | ++ | 10% CD4+90% CD8+No LH |
| 5 | 25 | M | Back | 0.2cm | 6 mo | Homogeneous structureless, FB | ++LaminarSuperficial | + | ++ | ++ | Yes | ++ | 10% CD4+90% CD8+LH |
| 6 | 66 | M | Chest | 2cm | 10 mo | Reticular/globular, structureless, P-L granules, FB | ++DelicateSuperficial | + | ++ | +++ | No | No | 30% CD4+70% CD8+No LH |
| 7 | 20 | M | Chest | 1cm | 11 mo | Homogeneous structureless, blood vessels, FB | +DelicateNot superficial | + | ++ | ++ | No | No | 50% CD4+50% CD8+No LH |
| 8 | 38 | F | Back | 0.6cm | 5 mo | Reticular structureless, P-L granules, FB | +DelicateNot superficial | ++ | ++ | + | No | No | 40% CD4+60% CD8+No LH |
| 9 | 38 | M | Chest | 0.4cm | 3 mo | Reticular/globular, structureless, P-L granules, FB | ++LaminarNot superficial | +++ | ++ | + | No | No | 30% CD4+70% CD8+No LH |
| 10 | 47 | F | Palm | 0.2cm | 8 mo | Parallel furrow, structureless | +DelicateNot superficial | + | 0 | + | No | No | No |
| 11 | 18 | M | Chest | 0.3cm | 10 mo | Globular, structureless, | ++LaminarNot superficial | + | + | +++ | No | No | 5% CD4+95% CD8+LH |
| 12 | 66 | F | Arm | 0.5cm | 5 mo | Reticular, structureless, P-L granules, FB | ++LaminarNot superficial | ++ | + | ++ | Yes | + | 10% CD4+90% CD8+No LH |
| 13 | 42 | F | Sole | 0.2cm | 3 mo | Parallel furrow, structureless | +DelicateNot superficial | 0 | 0 | + | No | No | No |
Abbreviations: F, female; FB, fibrosis (whitish); IHC, immunohistochemistry; LH, lentiginous hyperplasia; M, male; P-L, pepper-like.
The spontaneous regression of tumors is an enigma that has been described in nearly all types of cancer and often in both benign and malignant cutaneous melanocytic lesions, in which depigmentation makes regression more evident.20–25
Although partial regression is relatively common in melanoma,26–30 complete regression is very rare. Only 50 cases have been reported.7,8,14
Twice as many cases have been described in men as in women, and the mean age of the patients described has been 48 years. Lesions have ranged from 0.4cm to 3cm in size and 47% of patients have survived longer than 5 years.14 Complete regression has been considered to augur a poor prognosis in melanoma, but it is important to remember that nearly all the described cases occurred in tumors that had metastasized, at which point careful physical examination led to discovery of the regressed lesion that was suspected. If there have been melanomas that regressed spontaneously without metastasizing, such cases would be nearly impossible to confirm. One report described a patient with a melanoma of a Breslow depth of 0.7mm that had undergone complete spontaneous regression without metastasis 4 years after the patient refused to have it removed.14 Such cases suggest that the immune system might be able to destroy a melanoma quickly before the tumor is able to spread.
The halo nevus typifies the benign melanocytic nevus that is regressing. A halo nevus is characterized histologically by the progressive degeneration of melanocytes, which are destroyed by an inflammatory infiltrate that is very prominent initially and that gradually diminishes. By the final stage in the process, nevus cells are absent and only a moderate number of inflammatory cells remain.31–33 Although the etiology and pathogenesis of halo nevi have yet to be clearly determined, inflammation appears to be a precursor of regression, probably mediated by a cytotoxic mechanism involving CD8+ T cells.18,33–35 Halo nevi lacking a clinically visible halo have been described only rarely. This type of nevus is similar histologically to a conventional one.32A halo nevus develops as nevus cells disappear without the formation of fibrous tissue, distinguishing this lesion from a regressing melanoma, in which the disappearance of tumor cells is accompanied by papillary dermal fibrosis in the final stage.16,36 Attempts have been made to clarify the reasons for fibrosis in melanoma regression and the lack of it in halo nevus formation.16,36–38 Explanations have been sought in the fact that the expression of certain fibrogenic cytokines is more common in melanomas than in halo nevi, while halo nevi show much higher expression of tumor necrosis factor (TNF) α—a cytokine that inhibits fibrosis. Although the cytokine microenvironment does not seem to fully explain the development of fibrosis in melanoma regression (which is also related to interactions between the tumor, the stroma, and the inflammatory infiltrate), the elevated expression of TNF-α in halo nevi suggests that it plays an important role in inhibiting fibrosis in these lesions.16,36–38
In addition, the inflammatory infiltrate that leads to regression in melanoma is usually asymmetrical and patchy, affecting only parts of the tumor. The infiltrate of a regressing melanoma is not unlike that of a halo nevus, but the nevus has a larger number of CD8+ T cells while the melanoma has more CD4+ T cells.16,32,39–41
The clinical and dermoscopic features of the melanocytic nevi we analyzed revealed characteristic patterns. Clinically, these nevi involuted very rapidly. On histology, extremely thin collagen bundles were seen to have created a particular pattern of fibrosis referred to as delicate (Fig. 3). In all our cases, dermoscopy confirmed complete involution of the nevus within a much shorter period (mean of 6.4 months) than is observed in halo nevi. Loss of structure and the presence of multiple pepper-like granules (which is correlated with a histologic finding of melanophages) were the main dermoscopic findings (Fig. 8). In most cases, the morphologic features of the residual lesion were very similar to those seen in lichenoid keratosis. Although follow-up was not long in our series (ranging between 1 and 3 years), there were no cases of metastasis.
Certain characteristics suggest that the lesions we studied were not conventional halo nevi. The signs of regression also differed from those seen in melanoma regression. One difference was that none of the nevi in our series developed a clinically evident white halo. In addition, although it is common for patients to have several similar halo nevi, none of our patients had other regressing nevi and involution occurred in much less time than halo nevi take to form.
Immunohistochemistry confirmed the complete absence of nevus cells in these cases. Our observations included melanophages (Fig. 4), present in varying degrees and extensive in only 2 cases; inflammatory infiltrates (Fig. 5), mild or moderate in most cases; vascular proliferation (Fig. 3); and a particular type of fibrosis consisting of fine collagen bundles that were often located subepidermally (Fig. 3) and that have not been described in halo nevi or melanomas to date. In melanoma, fibrosis is usually dense but in halo-nevus formation it does not develop in the final stages. Fibrosis is generally a less significant finding in conventional nevi undergoing spontaneous regression than it is in regressing melanomas; an exception is the sclerosing nevus with pseudomelanomatous features that has recently been described.42–45 Such nevi are trizonal, consisting of an area of proliferating atypical melanocytes with pagetoid spread, an area of significant dermal sclerosis containing irregular nests of melanocytes, and an area of residual nevus tissue around and below the scar. Structural changes in these melanocytic neoplasms are usually so pronounced that they sometimes lead to a diagnosis of melanoma with regression or nevus-associated melanoma with regression. Nonetheless, the cytologic criteria essential for a diagnosis of melanoma are not met in most of these cases and to date complete regression has not been reported.
Delicate fibrosis, such as we observed in the nevi in this series, can be distinguished from the laminar fibrosis typical of a dysplastic nevus because parallel and/or concentric fibrotic bundles will not be present. Instead, thin collagen fibers will be scattered in the lesions and cellularity will be reduced and without signs of significant activation. In the absence of activation, the lesion can be considered stable and at the end of the fibrotic process, which will not continue on toward more organized patterns (as in a dysplastic nevus) or thickened bundles (as in melanoma regression). Therefore, we conclude that even though we removed the nevi within a very short period (in less than 2 weeks of noticing the changes), a biopsy performed at a later date would not have shown laminar fibrosis or thick bundles. The process would more likely have abated and the tissue would probably have been more similar to normal skin.
A melanoma in regression is usually accompanied by intense melanophage formation, and many authors consider nodular melanosis (extensive melanophage deposition) to be a key criterion for a diagnosis of complete melanoma regression.14 In our case series, however, the inflammatory infiltrates were less intense than is typical for halo nevi and the most common phenotype we observed was that of CD8+ T-cell predominance (Fig. 9), resembling halo nevi and different from a regressing melanoma (where CD4+ T cells predominate) (Table 4).
Main Criteria for the Differential Diagnosis of Completely Regressed Melanocytic Nevi.
| Melanoma | Halo Nevus | Our Cases | |
| Fibrosis | PresentCompactDeep | Absent | PresentDelicate-laminar |
| Inflammatory infiltrate | Variable intensityAssymetrical infiltrate | Very intenseSymmetrical infiltrate | Less intenseAssymetrical infiltrate |
| Lymphocyte phenotype | CD4+ T cells | CD8+ T cells | CD8+ T cells |
| Melanophages | Very dense | Variable density | Variable density |
| Blood vessels | Very dense | Variable density | Variable density |
| Atrophy | Frequent | Absent | Rare |
| Apoptosis | Variable | Marked | Rare |
The type of regression in this series most probably originated in an immune response to antigens expressed only by melanocytes in the affected nevus and not by those in the adjacent skin. These antigens would not have been widely expressed in other nevi on the patient's body, as happens in halo nevus formation and leads to events analogous to an explosion involving massive and extremely rapid destruction of tumor cells. The process we observed suggests a cytotoxic mechanism (confirmed in these cases by the marked predominance of CD8+ T cells) that was initiated by an inflammatory infiltrate acting very rapidly and disappearing early. The marked expression of major histocompatibility complex class I molecules by melanocytes makes them vulnerable to attack and destruction by cytotoxic T cells. Antigens that are expressed on the membrane of melanocytes and that are normally tolerated by the immune system probably undergo some type of change that makes them targets of these T cells. Once the cytotoxic T cells recognize the foreign antigens, they would induce cell death by mechanisms involving both FAS-ligand expression and direct damage to the cell membrane on release of perforin. Damage to the target cell may then amplify the immune response by releasing cytoplasmic antigens that would normally be inaccessible to the immune system.26,32
The delicate fibrosis observed would be explained by the rapidity of the regression process and by superficial repair.
The loss of dermoscopic structure in most lesions correlated with a finding of delicate or laminar fibrosis on histology. All lesions with multiple pepper-like granules on dermoscopy also showed moderate or intense presence of melanophages on histology. Vascular proliferation was the variable with the least correlation between dermoscopy and histology, as blood vessels were evident dermoscopically in only 7.6% of cases. A possible explanation is that we used a FotoFinder videocamera, which is used in contact with the skin, causing vasoconstriction.
ConclusionsIn recent years we have been able to identify a pattern of clinical and pathologic features peculiar to completely regressed melanocytic nevi.
The most remarkable clinical feature is the rapid rate of regression, which is characterized histologically by a particular type of delicate fibrosis that has not been described in melanoma to date.
When fibrosis on complete regression of a melanocytic nevus takes the form of very thin collagen fiber bundles and is found in addition to an inflammatory infiltrate in which cytotoxic T cells predominate, the evidence suggests that the involuted lesion was most likely benign.
Better understanding of the molecular mechanisms that lead to spontaneous regression of melanocytic nevi may help identify new therapeutic targets in melanoma.
FundingThis study was funded by a grant (AP-032/10) from the Department of Health of the Autonomous Community of Valencia.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
This study was awarded the Juan de Azúa Prize by the Spanish Academy of Dermatology and Venereology in 2011.
Please cite this article as: Martín JM, et al. Regresión complete de nevos melanocíticos: correlación clínica, dermatoscópia e histológica de una serie de 13 casos. Actas Dermosifiliogr.2012;103:401-10.