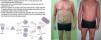
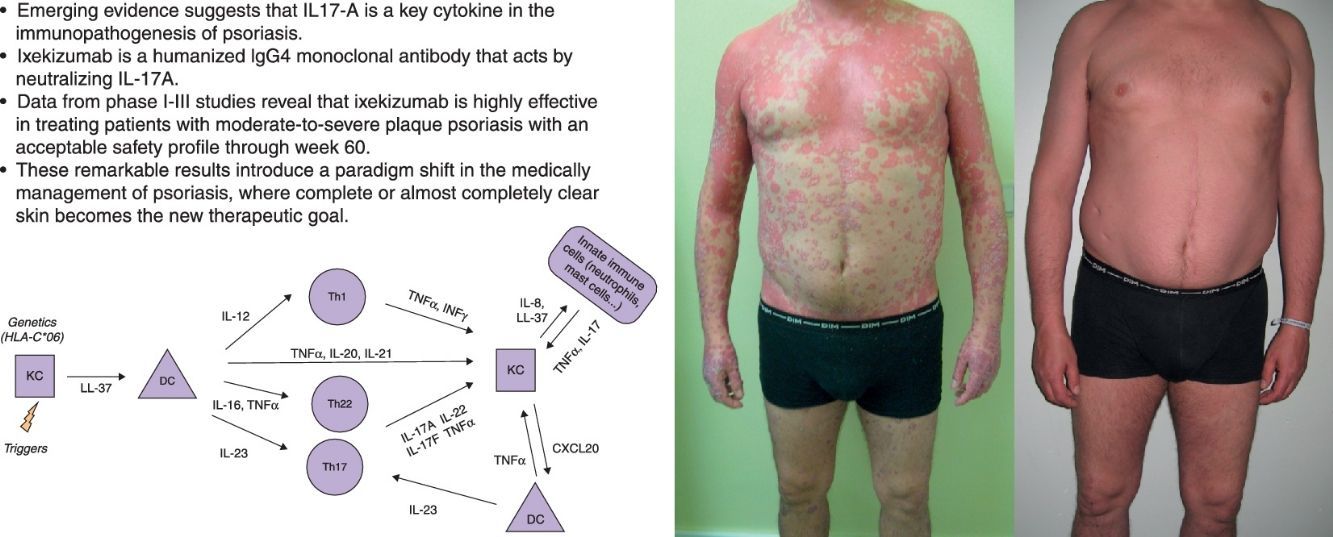
Psoriasis is a common, chronic, inflammatory skin disorder with a physical and emotional burden. Emerging evidence suggests that IL17-A is a key cytokine in the immunopathogenesis of psoriasis. Ixekizumab is a humanized IgG4 monoclonal antibody that acts by neutralizing IL-17A. Data from Phase I-III studies reveal that ixekizumab is highly effective in treating patients with moderate-to-severe plaque psoriasis. A large proportion of patients receiving ixekizumab achieved or maintained complete or near complete resolution of psoriatic lesions with an acceptable safety profile through week 60. These remarkable results introduce a paradigm shift in the medically management of psoriasis, where complete or almost completely clear skin becomes the new therapeutic goal.
La psoriasis es un trastorno cutáneo común, crónico e inflamatorio con una carga física y emocional. Las pruebas recientes sugieren que la IL17-A es una citocina clave en la inmunopatogénesis de la psoriasis. El ixekizumab es un anticuerpo monoclonal IgG4 humanizado que actúa neutralizando la IL17-A. Los datos de los ensayos fase III muestran una alta eficacia del ixekizumab para tratar pacientes con psoriasis en placas de moderada a grave. Una gran proporción de los pacientes que tomaba ixekizumab consiguió o mantuvo la resolución completa o prácticamente completa de las lesiones psoriásicas, con un perfil de seguridad aceptable en la semana 60. Estos notables resultados suponen un cambio de paradigma en la gestión médica de la psoriasis, donde una piel completamente o casi completamente libre de lesiones es el nuevo objetivo terapéutico.
Psoriasis is a chronic inflammatory skin disease affecting 1–3% of the adult population in western countries.1 About 20–30% of patients develop psoriatic arthritis and an association with an increased risk for cardiovascular disease and other cardiometabolic comorbidities has also been described.2,3
Treatment options include topical therapy, phototherapy and systemic agents such as retinoids, cyclosporine and methotrexate. However, a significant proportion of patients do not respond, are intolerant or have contraindications to these therapeutic options. Over the past few years, the introduction of biologic therapies targeting selective inflammatory mediators, namely TNF-α (adalimumab, etanercept and infliximab) and IL12/IL23p40 (ustekinumab), has improved treatment options for patients with moderate-to-severe psoriasis. Nonetheless there is still a subset of patients that do not respond to these biologic agents, lose treatment response over time or do not reach the highest clearance rate.4 These unmet needs lead to the development of biologic agents against other targets.4 Anti-IL17 drugs (including secukinumab and ixekizumab) are a promising therapy for psoriasis that act by neutralizing IL-17A, a key cytokine in the pathogenesis of the disease. This article aims to review the role of IL-17 in psoriasis immunopathogenesis and summarizes the data on efficacy and safety of ixekizumab.
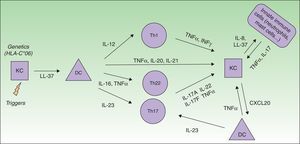
Biology of IL17Psoriasis is an immune-mediated disease initially considered to be a result of a Th1 response with the signature cytokines INF-γ and IL-12. However, recent studies investigating the molecular basis of psoriasis identified the key role of the adaptive Th17 cells.5
The actual model of immunopathogenesis (Fig. 1) proposes that an unknown antigen or environmental trigger activates macrophages, natural killer (NK) T cells and plasmacytoid dendritic cells (DCs) to secrete TNF-α, INF-α, INF-γ, IL-1β and Il-6, which trigger myeloid DCs activation. Activated myeloid DCs produce IL-12 and IL-23, leading to the differentiation of Th1 cells and Th17 cells, respectively.6,7 Th17 cells are important producers of IL-17A. Other cells, such as mast cells, neutrophils and NK cells, can also secrete IL-17A.8–10
IL-17A is a pro-inflammatory cytokine that belongs to the IL-17 cytokine family, which comprises six members (IL-17A-F).11 IL-17A,-C and-F expression is increased in psoriatic skin lesions.12 IL-17A shows similarities with IL-17F and both cytokines bind to the same receptor IL-17RA. The biologically active form of IL-17A comprises either an IL-17A homodimer or an IL-17A-IL-17F heterodimer, although the first one has greater biological activity.13,14
Serum levels of IL-17A are significantly increased in patients with psoriasis compared with healthy individuals and IL-17A positive T cells are found in elevated number in psoriatic lesions.15,16 The major pathogenic importance of the IL-23/Th17 axis over the IL-12/TH1 pathway is demonstrated by the higher expression of IL-23 in psoriatic skin lesions and its pathogenicity in inducing the psoriasiform phenotype in an experimental model injected with IL-23, but not with IL-12.17,18 Besides, the IL23/Th17 axis contributes to genetic susceptibility of psoriasis, linked to specific IL23p19 gene variants and to other genes such as the IL-23 receptor gene.19
IL-17A action includes: (i) activation of STAT3 signaling in keratinocytes, inducing their proliferation and expression of proinflammatory cytokines associated with skin inflammation; (ii) overexpression of antimicrobial peptides (AMPs), such as S100 proteins and β-defensins, which also have pro-inflammatory properties, (iii) secretion of several chemoattractants (CXCL1, CXCL3, CXCL5, CXCL6 and CXCL8) responsible for neutrophils accumulation in psoriatic epidermis; and (iv) increased expression of CCL20, which can attract CCR6-expressing DCs and T cells, creating a proinflammatory loop in the skin.20–24
Overview on IL-17 targeting agentsCurrently, there are three new biologic drugs targeting the IL-17A pathway used in clinical trials for psoriasis treatment. Ixekizumab is a humanized IgG4 IL-17A monoclonal antibody and secukinumab is a fully human IgG1 IL-17A monoclonal antibody, both acting by neutralizing IL-17A. Brodalumab is a human IgG2 monoclonal antibody that binds and blocks the IL-17RA receptor.
Secukinumab is already approved by FDA and EMA and it is commercially available in several countries, including Spain. Ixekizumab has been recently approved by FDA and EMA, and like secukinumab, is indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. The development of brodalumab was halted over concerns about suicidal ideation that could potentially lead to restrictive labeling.
Clinical efficacy of ixekizumabPhase IA 20-week randomized, double-blind, placebo-controlled, dose-escalation Phase I study was conducted in 40 patients with moderate-to-severe psoriasis.25 Patients were randomized into five groups receiving placebo or treatment—either 5,15, 50 or 150mg of subcutaneous ixekizumab—at weeks 0, 2 and 4.
The achievement of a 75% reduction of psoriasis area and severity index (PASI) at week 6 was significantly greater in the treatment groups receiving 15, 50 or 150mg of ixekizumab (25, 71 and 100%, respectively) compared with the placebo and the 5mg groups (0% for both groups) (p<0.001 for each comparison) (Table 1).
Clinical efficacy of ixekizumab in phase I trial.
| Study | Dosing (patients [n]) | Follow-up (weeks) | Results | |||
|---|---|---|---|---|---|---|
| Results assessment | PASI 75 (%) | PASI90 (%) | PASI100 (%) | |||
| Phase I triala,25 | 150mg (n=8) | 20 | Week 6 | 100 | 62.5 | NR |
| Week 20 | 74.1 | 74.1 | NR | |||
| 50mg (n=8) | 20 | Week 6 | 71.4 | 43 | NR | |
| Week 20 | 57.1 | 14.4 | NR | |||
| 15mg (n=8) | 20 | Week 6 | 25 | 12.5 | NR | |
| Week 20 | 50 | 50 | NR | |||
| 5mg (n=8) | 20 | Week 6 | 0 | 0 | NR | |
| Week 20 | 0 | 0 | NR | |||
| PBO (n=8) | 20 | Week 6 | 0 | 0 | NR | |
| Week 20 | 0 | 0 | NR | |||
A 20-week randomized, double-blind, placebo-controlled, dose-escalation Phase I study in which 40 patients with moderate-to-severe psoriasis were randomized into five groups receiving placebo (PBO) or treatment—either 5, 15, 50 or 150mg of subcutaneous ixekizumab—at weeks 0, 2 and 4.
Data note reported (NR); Psoriasis Area and Severity Index (PASI).
Biopsies collected at baseline, week 2 and week 6 showed a significant dose-dependent reduction from baseline in keratinocyte proliferation, hyperplasia, epidermal thickness, dermis and epidermis infiltration by T cells and DCs, and keratinocyte expression of AMPs, at week 2. Skin appeared normal at week 6.
Phase IIThe phase II trial was a 20-week randomized, double-blind, placebo-controlled study in which 142 subjects were randomized to placebo or treatment—10, 25, 75 or 150mg of ixekizumab—at 0, 2, 4, 8, 12 and 16 weeks.26
At week 12 the primary endpoint, PASI 75, was achieved in 76.7, 82.8 and 82.1% of the patients in the 25, 75, 150mg groups compared with 7.7% in the placebo group (p<0.001 for each comparison). At the same time point, a significant percentage of patients reached PASI 90 or PASI 100, in the higher doses groups. PASI 90 was observed in 58.6 and 71.4% of patients in the 75 and 150mg groups (p<0.001 vs. placebo). PASI 100 was observed in 37.9 and 39.3% of patients in the 75 and 150mg groups, respectively (p<0.001 vs. placebo) (Table 2).
Clinical efficacy of ixekizumab in phase II trials.
| Study | Dosing (patients [n]) | Follow-up (weeks) | Results | |||
|---|---|---|---|---|---|---|
| Results assessment | PASI 75 (%) | PASI90 (%) | PASI100 (%) | |||
| Phase II triala,26 | 150mg (n=28) | 20 | Week 12 | 82.1 | 71.4 | 39.3 |
| 75mg (n=29) | 20 | Week 12 | 82.8 | 58.6 | 37.9 | |
| 25mg (n=30) | 20 | Week 12 | 76.7 | 50.0 | 16.7 | |
| 10mg (n=28) | 20 | Week 12 | 28.6 | 17.9 | 0.0 | |
| PBO (n=26) | 20 | Week 12 | 7.7 | 0.0 | 0.0 | |
| Phase II open-label extensionb,27 | 120mg every 4 weeks (n=120) | 52 | Week 52 | 76.7 | 67.5 | 48.3 |
The phase II trial was followed by a 52-week open label extension (OLE) study, with patients receiving 120mg of ixekizumab every 4 weeks.27 Patients who did not reach PASI 75 at week 20 were immediately included in the extension study. Patients who achieved PASI 75 had a treatment-free period from week 20–32 and were included when they fell below PASI 75 or at week 32 if they maintained PASI 75 or higher. At week 52, among all patients who entered in the OLE, PASI 75 was observed in 77% of the patients, PASI 90 and PASI 100 were achieved in 68% and 48% of patients (Table 2).
Phase IIIThere are three large prospective, double-blind, multicentre, phase III studies (UNCOVER-1, -2 and -3) that evaluated ixekizumab efficacy and safety.
In UNCOVER-1, 1296 subjects were randomized to receive either placebo or ixekizumab 80mg every 2 or 4 weeks, after a 160mg starting dose, during 12 weeks (induction period).28,29 Responders to treatment (PASI 75 or sPGA=Static Physician's Global Assessment 0, 1) at week 12 were assigned to continue treatment on either placebo or ixekizumab (80mg every 4 or 12 weeks) for up to 60 weeks (maintenance period). PASI 75 at week 12 was achieved in 89.1 and 82.6% of the patients receiving ixekizumab every 2 weeks and 4 weeks, respectively, compared to 3.9% of the patients in placebo group (p<0.001).28 PASI 90 and PASI 100 response rates at week 12 were 70.9 and 35.3% in the ixekizumab every 2 weeks group; and 64.6 and 33.6% in the ixekizumab every 4 weeks (p<.001 vs. placebo).28 About 86–88% of patients considered responders at week 12 and treated with ixekizumab every 4 weeks (from week 12–60)maintained PASI 75 through week 60.29 And over 50% of responders maintained or reached complete clear skin at week 6029 (Table 3).
Clinical efficacy of ixekizumab in phase III trials.
| Study | Dosing or groups (patients [n]) | Follow-up (weeks) | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Results assessment | PASI 75 (%) | PASI90 (%) | PASI100 (%) | sPGA (0,1) (%) | sPGA (0) (%) | |||
| UNCOVER 1a | ||||||||
| Induction period | ||||||||
| IXE Q4W (n= 432) | 60 | Week 12 | 82.6 | 64.6 | 33.6 | 76.4 | 34.5 | |
| IXE Q2W (n= 433) | 60 | Week 12 | 89.6 | 70.9 | 35.3 | 81.8 | 37.0 | |
| PBO (n= 431) | 60 | Week 12 | 3.9 | 0.5 | 0.0 | 3.2 | 0.0 | |
| Maintenance period | ||||||||
| IXE Q4W→IXE Q12W (n= 110) | 60 | Week 60 | NR | NR | NR | 33.6 | 19.1 | |
| IXE Q4W→IXE Q4W (n=110) | 60 | Week 60 | 86.2 | NR | NR | 70.9 | 51.8 | |
| IXE Q2W→IXE Q12W (n=117) | 60 | Week 60 | NR | NR | NR | 41.0 | 21.4 | |
| IXE Q2W→IXE Q4W (n=119) | 60 | Week 60 | 87.9 | NR | NR | 74.8 | 54.8 | |
| IXE Q4W→PBO (n=109) | 60 | Week 60 | NR | NR | NR | 7.3 | 1.8 | |
| IXE Q2W→PBO (n=117) | 60 | Week 60 | NR | NR | NR | 7.7 | 3.4 | |
| UNCOVER 2b,30 | ||||||||
| IXE Q4W (n= 347) | 12 | Week 12 | 77.5 | 59.7 | 30.8 | 72.9 | 32.3 | |
| IXE Q2W (n= 451) | 12 | Week 12 | 89.7 | 70.7 | 40.5 | 83.2 | 41.9 | |
| ETN (n= 358) | 12 | Week 12 | 41.6 | 18.7 | 5.3 | 36.0 | 5.9 | |
| Study | Dosing or groups (patients [n]) | Follow-up (weeks) | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Results assessment | PASI 75 (%) | PASI90 (%) | PASI100 (%) | sPGA (0,1) (%) | sPGA (0) (%) | |||
| UNCOVER 2b,30 | ||||||||
| PBO (n= 168) | 12 | Week 12 | 2.4 | 0.6 | 0.6 | 2.4 | 0.6 | |
| UNCOVER 3b,30 | ||||||||
| IXE Q4W (n= 386) | 12 | Week 12 | 84.2 | 65.3 | 35.0 | 75.4 | 36.0 | |
| IXE Q2W (n= 385) | 12 | Week 12 | 87.3 | 68.9 | 37.7 | 80.5 | 40.3 | |
| ETN (n= 382) | 12 | Week 12 | 53.4 | 25.7 | 7.3 | 41.6 | 8.6 | |
| PBO (n= 193) | 12 | Week 12 | 7.3 | 3.1 | 0.0 | 6.7 | 0.0 | |
| UNCOVER Jc,34 | ||||||||
| Plaque psoriasis (n= 78) | 24 | Week 12 | 98.7 | 83.3 | 32.1 | |||
| Week 24 | 91.0 | 85.9 | 46.2 | |||||
| Erythrodermic psoriasis (n= 8) | 24 | Week 12 | 100.0 | 62.5 | 25.0 | |||
| Week 24 | 100.0 | 87.5 | 12.5 | |||||
| Generalized pustular psoriasis (n=5) | 24 | Week 12 | 80.0 | 60.0 | 20.0 | |||
| Week 24 | 80.0 | 40.0 | 40.0 | |||||
| Study | Dosing or groups (patients [n]) | Follow-up (weeks) | Results | ||
|---|---|---|---|---|---|
| UNCOVER 2b,30 | Results assessment | ||||
| Scalp psoriasis32 | PSSI100 (%) | ||||
| IXE Q4W (n= 312) | 12 | Week 12 | 64.1 | ||
| IXE Q2W (n= 320) | 12 | Week 12 | 74.4 | ||
| ETN (n= 322) | 12 | Week 12 | 44.7 | ||
| PBO (n= 151) | 12 | Week 12 | 7.3 | ||
| Palmoplantar psoriasis33 | PPASI75 (%) | PPASI100 (%) | |||
| IXE Q4W (n= 22) | 12 | Week 12 | 81.8 | 45.5 | |
| IXE Q2W (n= 31) | 12 | Week 12 | 74.2 | 51.6 | |
| ETN (n= 34) | 12 | Week 12 | 44.1 | 29.4 | |
| PBO (n= 18) | 12 | Week 12 | 16.7 | 5.6 | |
In UNCOVER-1, subjects were randomized to receive either placebo (PBO) or ixekizumab (IXE) 80 mg every 2 (Q2W) or 4 weeks (Q4W), after a 160 mg starting dose, during 12 weeks (induction period). Responders to treatment (PASI 75 or sPGA=Static Physician's Global Assessment 0, 1) at week 12 were assigned to continue treatment on either placebo or ixekizumab (80 mg every 4 [Q4W] or 12 weeks [Q12W]) for up to 60 weeks (maintenance period).
In UNCOVER-2 and -3, subjects were randomized to receive subcutaneous placebo, etanercept 50mg twice weekly or ixekizumab 80mg every 2 or 4 weeks after a 160mg starting dose, during a 12 week period.30 UNCOVER-2 included 1224 patients and UNCOVER-3 included 1346 patients. For UNCOVER-2 and -3, the primary endpoint, PASI 75 at week 12, was achieved, respectively, by 89.7 and 87. 3% of the patients in the ixekizumab every 2 weeks group, 77.5 and 84.2% of the patients in the ixekizumab every 4 weeks group, 41.6 and 53.4% of patients in the etanercept group and 2.4 and 7.3% of the patients in the placebo group (all p<0.0001 vs placebo or etanercept). For these 4 groups, PASI 90 response rates were, respectively, 70.7/68.1, 59.7/65.3, 18.7/25.7 and 0.6/3.1%, and PASI 100 response rates were 40.5/37.7, 30.8/35, 5.3/7.3, and 0.6/0% (all p<0.0001 vs placebo or etanercept) (Table 3).
About 24% of patients included in the UNCOVER -2 had prior experience with biologic therapies. PASI 75 response rates with ixekizumab were comparable between patients with prior exposure to biologic therapy and patients who were biologic naïve (93 and 89% in the every 2 weeks group and 74 and 79% in the every 4 weeks group, respectively) and markedly superior to etanercept and placebo groups (30/45% and 0/3%, respectively, p<0.001).31
Sub-analysis from UNCOVER-2 data showed that patients with scalp psoriasis receiving ixekizumab every 2 weeks reached on average 93% improvement in Psoriasis Scalp Severity Index (PSSI) at week 12 vs 70% in etanercept group (p<0.001) and 3.7% in placebo group (p<0.001).32 Complete resolution of scalp psoriatic lesions was observed in more than 60% of the patients in both ixekizumab dosing regimens at week 12 (p<0.001 vs. placebo, p<0.001 vs. etanercept).32 Sub-analysis of a subset of patients with moderate-to-severe palmoplantar psoriasis (n=105) included in UNCOVER-2 noted a 75% reduction of Palmoplantar Psoriasis Area and Severity Index (PPASI) at Week 12 in 82 and 74% of patients receiving ixekizumab every 2 weeks and every 4 weeks, respectively (p<0.001 vs. placebo, p<0.05 vs. etanercept).33 A 100% improvement in PPASI score was reached by 52% of patients in the ixekizumab every 2 weeks group compared to 5.6% in placebo group (p<0.001) and 29.4% in etanercept group (p<0.05).33
An open-label study (UNCOVER-J) included 91 Japanese patients with moderate-to-severe psoriasis (n=78), with erythrodermic psoriasis (n=8) and with generalized pustular psoriasis (n=5).34 Patients received 160mg ixekizumab as a starting dose followed by 80mg ixekizumab every 2 weeks through week 12 and every 4 weeks through week 24. At week 12, PASI 75, PASI 90 and PASI 100 response rates were 98.7, 83.3, and 32.1% in patients with plaque psoriasis. At week 24, patients maintained treatment efficacy (91, 85.9 and 46.2% of the patients with PASI 75, PASI 90 and PASI 100, respectively). All 8 patients with erythrodermic psoriasis and 4/5 (80%) of patients with generalized pustular psoriasis achieved PASI 75 at week 12 (Table 3). This study did not include a control group.
The efficacy of ixekizumab for treatment of patients with active psoriatic arthritis was assessed in a double-blind phase III trial (SPIRIT-P1).35 Patients naive to biologic therapy were randomized to subcutaneous placebo (n=106), adalimumab 40mg every 2 weeks (n=101), or ixekizumab 80mg every 2 weeks (n=103) or 4 weeks (n=107), after a 160mg starting dose. The primary endpoint, 20% improvement in the American College of Rheumatology response criteria (ACR20) at week 24, was achieved in 62.1% and 57.9% of patients in ixekizumab every 2 weeks or 4 weeks groups compared with 30.2% in the placebo group (p≤0.001). Efficacy results with adalimumab showed significant improvements versus placebo. Both ixekizumab groups showed improvement in disease activity (p≤0.001) and functional disability (p≤0.01) versus placebo at week 24. Progression of structural damage was significantly less in the two ixekizumab and adalimumab groups compared to the placebo group (p≤0.01) at week 24. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
SafetyA point of concern is the finding that patients with deficiencies in the IL-17 pathway have a high risk of developing chronic mucocutaneous candidiasis.36 In the phase II trial and in the open-label extension study no cases of fungal infections were reported.26,27 In UNCOVER-2 and -3, candida spp infections were higher in patients receiving ixekizumab (12 patients receiving ixekizumab every 2 weeks, 4 patients receiving ixekizumab every 4 weeks vs 5 patients receiving etanercept and 2 patients receiving placebo).30 In UNCOVER-1, during the induction and maintenance period, more cases of candida infections were reported in patients receiving ixekizumab (during the induction period, 3 patients in the ixekizumab every 4 weeks group and 4 patients in the ixekizumab every 2 weeks group vs. 2 patients in the placebo group, during the maintenance period, 4 patients in the ixekizumab every 12 weeks group and 8 patients in the ixekizumab every 4 weeks vs.2 patients in the placebo group).28 All infections resolved with standard treatment, no patient needed to discontinue the study drug and no invasive fungal infection was reported.28,30
The role of IL-17A in the differentiation and maturation of neutrophil granulocytes lead to a concern about the development of neutropenia in patients receiving IL-17A inhibitors.37 In the phase II study, asymptomatic grade 2 neutropenia occurred in 2 patients receiving 75 and 150mg of ixekizumab.26 In the open-label extension study, no effect on mean neutrophil count was observed in patients with prolonged ixekizumab treatment.27 In UNCOVER-J, grade 1 neutropenia was observed in 15 patients and grade 2 neutropenia in 4 patients.34 In UNCOVER-2 and -3, grade 3 neutropenia was found in 2 patients receiving ixekizumab every 2 weeks, 4 patients receiving etanercept and 1 patient receiving placebo. Grade 4 neutropenia was observed in 1 patient receiving ixekizumab every 4 weeks.30 However, no patients with grade 3 or 4 neutropenia had a concurrent infection.30
Previous clinical trials with anti-IL17 drugs in patients with Crohn's disease (CD) suggested worsening of the disease and no evidence of clinical efficacy with IL-17A inhibition.38,39 Among 3858 patients included in UNCOVER-1, -2 and 3, the occurrence of CD (2 cases, 1 case in ixekizumab every 2 weeks group and 1 case in ixekizumab every 4 weeks group) and ulcerative colitis (2 cases, both in ixekizumab every 2 weeks group) was uncommon during the induction period.40
Integrated data from 5 phase III clinical trials (UNCOVER-A, -J, -1, -2 and -3) demonstrated that Major Adverse Cardiac Events (MACE) were uncommon and balanced across treatment groups.41 The incidence rate of MACE stayed stable over time among patients treated with ixekizumab, suggesting that ixekizumab is not associated with an increased risk of MACE.41
Regarding the increased risk of suicidal ideation, that has been associated with brodalumab, available data do not support an association of ixekizumab with suicidal behaviour.42
No cases of tuberculosis reactivation were reported in the phase II and III studies.26,30,34
The most common reported adverse events (AEs) in the phase II and III studies were nasopharingitis, upper respiratory infection, injection sites reactions and headaches.26,30 There were no severe AEs observed in the phase II study.26 In the UNCOVER 2-3, serious AEs were reported in less than 2% of patients across all study groups.30 In these studies, no clear difference was seen in rates of serious infections or malignancies among patients receiving placebo and those receiving ixekizumab. Patients who reached complete or almost complete clear skin did not appear to be at increased risk for infections or severe AEs.43 This finding is observed in patients treated either with ixekizumab or etanercept during 12 weeks.43
Immunogenicity is an issue of concern with the use of monoclonal antibodies. In UNCOVER-1,-2 and -3, at week 12, neutralizing anti-drug antibodies (ADA) were observed at low titers in 5.7% and 8.0%, at moderate titers in 1.6% and 3.0% and at high titers in 1.7% and 2.4% of patients receiving ixekizumab every 2 weeks and every 4 weeks, respectively.44 Only high ADA titers (≥1280) were associated with reduced efficacy. In UNCOVER-1 and -2, at week 60, ADA were reported at low titers in 14.2% and at moderate titers in 0.9% of patients receiving ixekizumab every 2 weeks re-randomized to ixekizumab every 4 weeks.44 None of these patients had high ADA titers. Efficacy was similar between negative ADA patients and patients with low and moderate ADA titers at week 60. No association between ADA and treatment AEs was detected.44
DiscussionPsoriasis is a chronic, debilitating skin disorder that has a negative impact in health-related quality of life. Recent evidence supports that IL-17 is a key mediator in the pathogenesis of psoriasis. A new class of biologic agents, which includes secukinumab and ixekizumab, was designed to inhibit IL-17A action. Data from Phase I-III studies has demonstrated a high efficacy of ixekizumab for treatment of moderate-to-severe psoriasis with 78–90% of the patients achieving PASI 75 at week 12. The two large phase III studies demonstrated that ixekizumab not only had greater efficacy over placebo but also over etanercept, a widely used anti-TNFα biologic agent. A greater proportion of patients receiving ixekizumab achieved PASI 90 (60–71% of the patients) and PASI 100 (31–41% of the patients), which was markedly superior to the results of etanercept treatment (19–26% and 5–7% of patients, respectively). Moreover, the findings of phase II and III trials showed that patients who achieved 90–99% or 100% PASI improvement had significantly greater enhancement in health related quality of life and pruritus symptom relief compared with patients with lesser improvement in PASI.30,45 This finding encourages the upgrade of the treatment goals to PASI 90 or 100, with the consequently positive impact in patient's quality of life.46 Higher clearance rates were not associated with increased risk for infections or serious AEs.43
These promising results at short term appeared to be maintained at long term as demonstrated in UNCOVER-1, as 86-88% of responder patients maintained PASI 75 and over 50% maintained or reached complete clear skin at week 60.
Ixekizumab therapy also demonstrated improvement in scalp and palmoplantar psoriasis.
Additionally, ixekizumab showed high efficacy in the treatment of psoriatic arthritis with reduction of signs and symptoms and inhibition of bone destruction. Even though no head-to-head studies have been performed, results for ixekizumab (58–62% of patients with ACR20) seem to be slightly higher than secukinumab (51–54% of patients with ACR20) in patients with psoriatic arthritis at week 24.35,47 So, it is expectable that ixekizumab becomes useful in the management of this patients, in the same way of secukinumab, which was already approved for treatment of psoriatic arthritis. Lxekizumab showed a safety profile at week 12 comparable with other anti-IL17 agents and etanercept. There is a concern about the risk of development of mucocutaneous candidiasis and neutropenia. So far, these AE were infrequently observed, mild or moderate in severity and did not lead to discontinuation of the study drug. Clinical monitoring for skin and mucosal infections and laboratorial monitoring of neutrophils blood count may be advisable during ixekizumab treatment.
Psoriasis patients are approximately 2.5-times more likely to have CD and 1.6-times more likely to have ulcerative colitis compared with age- and sex-matched controls.48 Previous studies with brodalumab and secukinumab revealed that blockade of IL-17A did not improved CD and can even result in disease worsening.38,39 An alteration of gut mycobiome, with Candida albicans proliferation secondary to loss of control by IL-17, may play a role in worsening of CD.49 The incidence of inflammatory bowel disease in patients receiving ixekizumab was low during the first 12 weeks. Still it is important to be alert for symptoms and signs of inflammatory bowel disease (IBD) in patients receiving ixekizumab. Ixekizumab and other anti-IL17 drugs should be avoid in patients with a diagnosis of IBD.
The humanization process used to generate ixekizumab allows optimization of its affinity for IL-17A, resulting in a molecule with a very high binding affinity.50 This results in high potency in blocking the IL-17A-mediated biological activities in vitro and in vivo.50 Unlike secukinumab, ixekizumab is a humanized IgG4 monoclonal antibody and retain a murine-derived sequence, which can raise the risk of immunogenicity. Still, IgG4 is thought to be less immunogenic than IgG1 molecules as secukinumab because it does not activate the classic complement system.51 Human or humanized antibodies still have potential for immunogenicity due to unique complementarity-determining regions (CDR).52 During inducing treatment with ixekizumab, only a small number of patients (1.7–2.4%) develop high ADA titers, which are associated with reduced efficacy. During maintenance treatment among initial responder patients, ixekizumab revealed low immunogenicity with no loss of efficacy. Although there is no direct comparison, secukinumab appears to be moderately superior in this point, with minimal immunogenicity up to Week 12 (ADA in 0% to 0.41%. of patients).53–57 Secukinumab maintained a low rate (0.4%) of treatment-emergent ADA up to Week 52, across six phase 3 clinical trials.58 Furthermore, there is no report of loss of secukinumab efficacy.58
Both ixekizumab and secukinumab have impressive results that have never been achieved with previous therapeutics. Even though there are no direct comparisons available, small details may make a difference in the choice between ixekizumab and secukinumab. Reported efficacy for ixekizumab is apparently superior to secukinumab efficacy for treatment of psoriasis30,53. Conversely, immunogenicity data favors secukinumab. Differences in the posology of these two anti-IL17 drugs may affect treatment adherence. Ixekizumab shows a more favorable posology at long term with only one injection for each monthly dose vs. two injection of secukinumab for each monthly dose.
For the first time, two biological drugs have been approved as first line therapy for systemic treatment in moderate to severe psoriasis. This decision reflects the remarkable efficacy associated with a favorable safety profile of ixekizumab and secukinumab in clinical trials. Both may provide an answer to the unmet needs of patients who did not respond to or did not tolerate previous treatments. Notably, anti-IL-17 drugs may initiate a new era in the psoriasis treatment, where PASI 90 or 100 becomes the new therapeutic goal. The next years may clarify the possible economic burden on healthcare systems by the use of these anti-IL-17 drugs as first line systemic therapies for psoriasis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestT. Tiago has participated in clinical trials sponsored by AbbVie, Amgen and Novartis and has received honoraria for acting as a consultant and/or as a speaker at events sponsored by AbbVie, Boehringer Ingelheim, Janssen, Leo-Pharma, Lilly, MSD, Novartis and Pfizer. A. Azevedo has no conflicts of interest to disclosure.