Topical clascoterone (TC) is the first topical antiandrogen approved by the Food and Drug Administration (FDA) for the treatment of acne and is currently under evaluation for approval in Europe. Through bibliographic searches in Medline and Google Scholar, we conducted a narrative review to assess the usefulness of TC in acne management. Several randomized clinical trials have demonstrated its efficacy and safety (with virtually no systemic adverse effects), but few have compared its effectiveness with other topical agents or its use in combination. It is a highly interesting therapeutic alternative, particularly for patients who do not tolerate other topical treatments or in cases of acne with a strong hormonal component. U.S. treatment guidelines conditionally recommend it for acne management due to its high cost. The strength of recommendation is lower than that of topical retinoids and benzoyl peroxide.
Acne is a very common chronic inflammatory disease of the pilosebaceous unit that affects children, adolescents, and adults, with an estimated incidence rate of 85% among adolescents and young adults in Spain.1,2 It can have a significant impact on quality of life.3,4 Its pathophysiology includes increased androgen-mediated sebum production, an impaired follicular keratinization, colonization by Cutibacterium acnes, and an inflammatory response involving both innate and adaptive immunity.5,6 Treatment of mild-to-moderate forms is based on topical agents such as retinoids, benzoyl peroxide, and combinations with antibiotics.7 For moderate-to-severe cases, systemic treatments such as tetracyclines and isotretinoin are used, and in women, various antiandrogenic agents such as spironolactone and oral contraceptives.8,9 In addition, over the past decade, new topical and systemic agents have emerged (Table 1), notably topical clascoterone (TC) 1% cream (Winlevi®), which represents the first topical antiandrogen available. In 2020, the US Food and Drug Administration (FDA) approved its use for patients aged≥12 years with acne vulgaris.10,11
Topical and systemic drugs approved in recent decades for acne management.
| Route of administration | Drug name | Year of FDA approval | Approved by EMA | Indication |
|---|---|---|---|---|
| Topical | Tazarotene 0.1% foam | 2012 | No | Mild-to-moderate acne |
| Trifarotene 0.005% cream | 2019 | No | Moderate acne | |
| Minocycline 4% foam | 2019 | No | Moderate-to-severe acne | |
| Clascoterone 1% cream | 2020 | No | Moderate-to-severe acne | |
| Systemic | Sarecycline | 2018 | No | Moderate-to-severe acne |
| Micronized isotretinoin | 2019 | No | Severe acne | |
FDA, Food and Drug Administration; EMA, European Medicines Agency.
The aim of this review is to assess the effectiveness and safety of TC and define its positioning within international therapeutic clinical practice guidelines.
Materials and methodsWe conducted a narrative literature review in January 2025. The search was conducted across MEDLINE and Google Scholar using the following keywords: “acne,” “clascoterone,” “antiandrogenic,” “treatment,” “guidelines.” Articles in Spanish and English were included. Clinical trials, meta-analyses, systematic reviews, and treatment guidelines were selected. Articles were initially screened based on title and abstract and were subsequently selected according to relevance after full-text review. All 3 authors participated in the search and selection process. Additionally, ongoing clinical trials were identified through the ClinicalTrials.gov database.
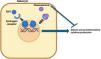
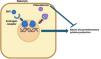
ResultsMechanism of action and dosingClascoterone, or cortexolone 17α-propionate, is an ester derivative of cortexolone with a chemical structure similar to spironolactone and dihydrotestosterone (DHT). This similarity allows it to competitively bind androgen receptors, reducing the activation of androgen-dependent genes involved in acne-related inflammation and sebum production, without the need to inhibit 5α-reductase (Fig. 1).12–16 The scope of action of clascoterone is limited to the areas of application, as it is hydrolyzed within the epidermis to cortexolone.5,14 This metabolite is a precursor of endogenous glucocorticoid synthesis, with minimal intrinsic glucocorticoid activity and no clinically relevant endocrinological effects.5
Mechanism of action of clascoterone. Dihydrotestosterone (DHT) binds to androgen receptors (ARs) within the sebocyte cytoplasm, subsequently dimerizing and translocating into the cell nucleus, where it stimulates transcription of androgen-dependent proinflammatory genes that ultimately increase sebum production. Clascoterone competitively inhibits ARs, preventing DHT from binding to these receptors. As a result, the production of proinflammatory cytokines and sebum is reduced.
CT is applied as a thin layer over acne lesions (approximately 1g of cream) every 12h.17
Effectiveness in the treatment of acneThe efficacy profile of TC in acne has been evaluated in several randomized clinical trials (RCTs), mostly placebo-controlled (Tables 2 and 3). In 2011, a double-blind RCT included 77 men with facial acne, an Investigator's Global Assessment (IGA) score of 2 or 3, and a total lesion count (TLC) of 20–100. Three groups were compared: TC 1%, tretinoin 0.05%, and placebo for 8 weeks. TC was more effective than placebo (primary endpoint) at all study timeframes (weeks 2, 4, 6, and 8), achieving a significant reduction in TLC (65.70%±31.42 vs 37%±33.31; P=.0017), and better outcomes in inflammatory lesion count (ILC) and Acne Severity Index (ASI) (P=.0134 and P=.009, respectively). Compared with tretinoin (secondary endpoint), TC showed better results, reaching statistical significance only for reduction in ILC at week 6 (P=.037). Time to achieve a 50% improvement in TLC, ILC, and ASI was shorter with CT.16
Published studies on the efficacy profile of topical clascoterone.
| Authors and year | Sample size | Study type | Primary objective | Treatment arms (number of patients) | Main results |
|---|---|---|---|---|---|
| Trifu et al.16 2011 | 77 | Phase I clinical trial | To evaluate the safety and efficacy of TC 1% vs placebo | – TC 1% (n=30)– Tretinoin 0.05% (n=32)– Placebo (n=15)All treatments applied once daily for 8 weeks | CT 1% vs placebo:– Significant reduction in TLC and NILC in favor of TC 1%.CT 1% vs tretinoin 0.05% (Table 3) |
| Mazzetti et al.18 2019 | 363 | Phase 2b clinical trial | To identify the most effective and safest dose and regimen of CT | – TC 0.1% q12h (n=72)– TC 0.5% q12h (n=76)– TC 1% q24h (n=70)– TC 1% q12h (n=70)– Placebo q12h or q24h (n=75)All treatments for 12 weeks | The most effective option was TC 1% q12h, with no safety differences vs other regimens or placebo |
| Hebert et al.12 2020 | 1440 | 2 phase 3 clinical trials (CB-03-01/25 and CB-03-01/26) | To evaluate the safety and efficacy profile of TC 1% vs placebo | – TC 1% (n=722)– Placebo (n=718)All treatments applied q12h for 12 weeks | Statistically significant differences in favor of TC 1% in reduction of TLC, ILC, NILC, and improvement in IGAa |
| Eichenfield et al.19–21 2020, 2023, 2024 | 609 | Open-label, long-term extension trial (CB-03-01/27) | To evaluate the safety and efficacy profile of TC 1% after 9 months of treatment | – TC 1% q12h×9 months (n=609) | 48.9% and 52.4% of patients achieved a significant target IGAa improvement in facial and truncal acne, respectively |
| Alkhodaidi et al.22 2021 | 2475 | Systematic review and meta-analysis | To evaluate the safety and efficacy profile of TC 1% | – TC 1% (n=1357)– Placebo (n=1118) | RR for treatment success (IGAa improvement): 95%CI, 2.11–3.89 in favor of CT |
Studies evaluating the efficacy of TC vs other drugs.
| Authors and year | Sample size | Study type | Objectives | Treatments evaluated | Main results |
|---|---|---|---|---|---|
| Trifu et al.16 2011 | 77 | Phase I CT with direct comparison | As a secondary endpoint, to evaluate the safety and efficacy profile of TC 1% vs tretinoin 0.05% | – TC 1% (n=30)– Tretinoin 0.05% cream (n=32)– Placebo (n=15)All treatments applied once daily for 8 weeks | CT 1% vs tretinoin 0.05%:– Significant reduction in ILC with TC 1%.– No statistically significant differences in TLC reduction or IGAa improvement |
| Basendwh et al.29 2024 | 2006 | Systematic review and network meta-analysis with indirect comparison | To evaluate the safety and efficacy profile of TC and spironolactone | – TC 0.05% q12h– TC 0.1% q12h– TC 1% q12h– TC 1% q24h– Spironolactone 25mg q24h– Spironolactone 50mg q24h– Spironolactone 200mg q24h | – TLC reductionb: Spironolactone 200mg was significantly superior to TC 1%. No differences between TC 1% and other spironolactone doses.– ILC reductionb: TC 1% was significantly superior to spironolactone 50mg. No differences vs other spironolactone doses |
| Shergill et al.30 2024 | 5474 | Systematic review and meta-analysis with indirect comparison | To evaluate the safety and efficacy profile of CT, trifarotene, and tazarotene | – TC 1%– Trifarotene 0.005% cream– Tazarotene 0.045% lotion | No statistically significant differences among the three treatments in IGAa improvement, ILC, or NILC |
In 2019, a phase 2b RCT including 363 patients (53% women) compared TC applied every 12h or every 24h at increasing concentrations vs placebo. The most effective regimen was TC 1% applied every 12h.18 In 2020, 2 phase 3 RCTs (CB-03–01/25 [n=722] and CB-03-01/26 [n=718]) evaluated the safety and efficacy profile of TC 1% every 12h vs placebo for 12 weeks.12 These trials included a total of 1440 patients aged≥9 years with moderate-to-severe acne (IGA 3 or 4). Approximately 20% of CT-treated patients achieved an IGA≤1 vs 6–9% in the placebo group (P<.001). Furthermore, TC significantly reduced ILC and NILC (non-inflammatory lesion count), achieving a 38% reduction in TLC vs 22–28% with placebo (P<.001).12 Moreover, an open-label, long-term extension study (CB-03-01/27) evaluated TC 1% every 12h for an additional 9 months. This study included a total of 609 patients aged≥12 years, 343 of whom completed treatment.19–21 At study end, 48.9% and 52.4% of patients achieved an IGA≤1 for facial and truncal acne, respectively.19–21
In 2021, a meta-analysis by Alkhodaidi et al. (n=2475 patients) reported that TC significantly increased the likelihood of treatment success (defined as achieving IGA≤1 or a ≥2-point reduction in IGA) vs placebo at week 12 (RR, 2.87; 95%CI, 2.11–3.89). TC also demonstrated significant reductions in ILC (mean difference [MD], −5.64) and NILC (MD, −1.82).22
Currently, a recruiting RCT (NCT0589179523) is evaluating the efficacy of TC in steroid-induced acne in transgender patients on masculinizing hormone therapy. Another active RCT (NCT0641530524) is assessing its use in patients with darker skin types. Regarding combination therapy, a completed phase IV trial (NCT0633662925) evaluated TC+benzoyl peroxide+clindamycin, although results are not yet available, and another recruiting study (NCT0633660326) is assessing its combination with adapalene 0.3%.
In real-world clinical practice, 2 studies have been identified.27,28 The first, by Lynde et al.,27 reported a case series of 9 patients treated with TC either as monotherapy or in combination with other therapies (including spironolactone, oral isotretinoin, or topical retinoids) in various clinical scenarios, notably a transgender patient with hormonally induced acne and another patient with acne and atopic dermatitis. The second study, by Tay et al.,28 described 10 individuals treated with TC alone or in combination, including a patient on masculinizing therapy in whom TC use was associated with favorable clinical outcomes. No adverse events were observed among the 19 patients, and both studies concluded that TC use proved safe and effective.
Clascoterone vs other topical treatmentsTwo systematic reviews and meta-analyses comparing TC with other topical treatments were identified (Table 3). Basendwh et al.29 included a total of 7 studies with 2006 patients up to June 2022 and indirectly compared spironolactone with CT. Spironolactone at 200mg/day was the most effective treatment for reducing TLC, followed by TC 1%. For ILC reduction, only comparisons between TC 1% and spironolactone 25mg were available, with TC 1% showing greater efficacy.
The second systematic review and meta-analysis by Shergill et al.30 included a total of 6 articles and indirectly compared TC (n=722) with trifarotene 50μg/g cream (n=1214), tazarotene 0.045% lotion (n=799), and placebo (n=2739). The authors found no significant differences among between trifarotene, tazarotene, and TC in reducing ILC and NILC. Similarly, no significant differences were observed in treatment success, defined as a ≥2-point improvement in IGA or evaluator's global severity score.
Safety profileBoth in phase I16 and in phase II18 and phase III19 clinical trials, adverse events (AEs) were mild or moderate and resolved completely by the end of the study. A small number of cases of hyperkalemia were reported, although these were not clinically significant. However, following evaluation by the US Food and Drug Administration (FDA), no association was found between plasma levels of clascoterone or cortexolone and hyperkalemia.31 In a phase I clinical trial, TC demonstrated better tolerability than tretinoin with respect to cutaneous irritation, which was evident from the first study visit.16 No serious AEs or patient AE-related discontinuations were reported. In phase III clinical trials (CB-03-01/25 and CB-03-01/26), most reported AEs were mild, with local irritation being the most common, and no significant differences vs placebo were observed.12 In the long-term extension study CB-03-01/27, no serious AEs were observed after 9 months of treatment.21 No study reported signs of feminization (e.g., gynecomastia) or decreased libido.
Regarding the hypothalamic–pituitary–adrenal (HPA) axis, 2 phase II clinical trials assessed adrenal function using the adrenocorticotropic hormone (ACTH) stimulation test. Only 5 of 69 patients on TC showed abnormal results at day 14, which normalized after repeat testing at 4 weeks.18,32
Finally, no studies have evaluated interactions between TC and other drugs or its safety in pregnant or breastfeeding women.14
Positioning in acne treatment guidelinesEight international acne management guidelines were reviewed,33–39 including North American, European, and Asian guidelines (Table 4). TC is mentioned only in the most recent US clinical practice guidelines, published in 2024.9 In these guidelines, TC is supported by a high level of evidence; however, only a conditional recommendation was issued due to its high cost. Recent publications estimate monthly costs of approximately US$300–600 in the United States.40,41
Positioning of TC treatment in international acne treatment guidelines.
| Authors and year | Country of origin | Recommendation regarding TC |
|---|---|---|
| Reynolds et al.9 2024 | United States | Conditional recommendation (due to high treatment cost). This conditionality will be reviewed depending on changes in cost and access to treatment. High level of evidence supporting TC |
| Qing Ju et al.33 2019 | China | Not mentioned |
| Oon et al.34 2019 | Singapore | Not mentioned |
| Thiboutot et al.35 2018 | International | Not mentioned |
| Hayashi et al.36 2017 | Japan | Not mentioned |
| Le Cleach et al.37 2017 | France | Not mentioned |
| Asai et al.38 2016 | Canada | Not mentioned |
| Nast et al.39 2012 | Europe | Not mentioned |
The treatment of acne can be complex due to the wide variety of lesion types, patient sex and comorbidities, variability in quality-of-life impact—even in mild acne—and the tolerability and effectiveness of available therapies.42
Current practice guidelines recommend topical retinoids, either as monotherapy or in combination, as first-line therapy for mild-to-moderate acne.9 These agents have demonstrated effectiveness not only in reducing ILC and NILC, but also in decreasing scarring, hyperpigmentation, and postinflammatory erythema (PIE).9,43,44 However, they may cause significant cutaneous irritation, which can compromise treatment adherence.9,38 A similar issue occurs with benzoyl peroxide.45 Conversely, topical antibiotics are not recommended as monotherapy, nor is prolonged use of systemic antibiotics.9 Although isotretinoin and antiandrogenic agents have a favorable safety profile, they are not exempt from adverse effects.46 In addition, a non-negligible proportion of patients are reluctant to receive systemic therapy. In this context, TC emerges as a well-tolerated (with very few topical adverse effects) and effective alternative. Its use is supported by multiple clinical trials (phase I, II, III, and extension studies), as well as by meta-analyses and systematic reviews. TC should be considered for facial or truncal mild-to-moderate acne, particularly in patients with poor tolerance to topical retinoids (as monotherapy or in combination) and/or those who do not wish to receive systemic medication.
Real-world data on TC use remain scarce but support its potential use in combination with other therapies and across different acne subtypes.27,28 Furthermore, due to its pathophysiological mechanism of action, TC may be especially useful in individuals with acne with a significant hormonal component, such as those with polycystic ovary syndrome, although no studies have specifically evaluated its efficacy in distinct phenotypes beyond the aforementioned case series.27,28 Similarly, no studies have assessed its efficacy in reducing acne scarring or PIE. Cost represents another potential limitation, as illustrated by a cost-effectiveness analysis conducted by Canada's Drug Agency, which did not recommend reimbursement.47 An additional limitation is the lack of availability in Spain, although approval by the European Medicines Agency (EMA) is expected imminently, with the market price still unknown. In the United Kingdom, TC has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) since early February 2025 and is already commercially available.
Spironolactone and combined oral contraceptives containing antiandrogenic progestins are well-tolerated therapeutic options with good response rates and are recommended in acne management guidelines.9 However, they should not be prescribed to men due to the risk of gynecomastia and other AEs.48 In women, despite good tolerability, these therapies may cause menstrual irregularities and other systemic symptoms, leading some patients to decline their use. In this setting, TC represents a valuable alternative, as it offers a more favorable safety profile. Despite being an antiandrogenic agent, its topical formulation and mechanism of action avoid the systemic adverse effects associated with systemic antiandrogens.12,21
Regarding the comparative efficacy of TC vs other topical agents, evidence remains limited and is largely based on indirect comparisons, which constitutes a major limitation for interpreting results.29,30 To date, only 1 randomized controlled trial comparing TC with tretinoin has been identified.16 This study concluded that TC achieved a faster and more favorable therapeutic response and was associated with fewer local AEs than the retinoid.19
LimitationsThis review is limited by its narrative design, as it is neither a systematic review nor a meta-analysis. In addition, very few studies directly compare TC with other topical therapies (e.g., benzoyl peroxide, topical retinoids, or antibiotic-containing topical combinations), making it difficult to determine superiority among treatments. Real-world data on the safety and efficacy profile of TC are also limited. Furthermore, no cost-effectiveness studies of TC have been conducted in Europe.
ConclusionsTC is the first topical antiandrogenic treatment available for acne, without clinically significant systemic adverse effects, and can be used in both women and men. Its effectiveness and safety are supported by multiple randomized controlled trials. However, further studies are needed to evaluate its efficacy in combination with other topical acne treatments, as well as to provide direct comparative data with alternative therapeutic options. Currently, TC is considered a promising option in acne cases where the hormonal component plays a major role and in situations where other topical therapies fail to achieve adequate efficacy or are poorly tolerated. Nonetheless, its high cost and lack of availability in Spain remain significant barriers to its widespread use.
Conflict of interestThe authors declare that they have no conflict of interest.