The Spanish Mohs Surgery Registry is used to collect data on the use and outcomes of Mohs micrographic surgery (MMS) in Spain. The aim of this study was to describe perioperative and intraoperative data recorded for MMS procedures performed between July 2013 (when the registry started) and January 2016.
Material and methodsProspective cohort study of data from 18 hospitals. The data collected included type of anesthesia, surgical technique, hospital admission, number of Mohs stages, management of preoperative risk factors, additional treatments, previous treatments, type of tumor, operating time, and complications.
ResultsData were available for 1796 operations. The most common tumor treated by MMS was basal cell carcinoma (85.96%), followed by squamous cell carcinoma (6.18%), lentigo maligna (2.81%), and dermatofibrosarcoma protuberans (1.97%). Primary tumors accounted for 66.9% of all tumors operated on; 19.2% of tumors were recurrent and 13.9% were persistent. The most common previous treatment was surgical. MMS was mostly performed under local anesthesia (86.7% of cases) and as an outpatient procedure (71.8%). The frozen section technique was used in 89.5% of cases. One stage was needed to achieve tumor-free margins in 56.45% of patients; 2 stages were required in 32.1% of patients, 3 in 7.1%%, 4 in 2.7%, and 5 or more in 1.8%. The defect was reconstructed by the dermatologist in 98% of patients and the most common technique was flap closure (47.2%). Intraoperative complications were recorded for just 1.62% of patients and the median (interquartile range) duration of surgery was 75 (60-100) minutes.
ConclusionThe characteristics of the patients and tumors treated by MMS are similar to those reported for similar studies in other geographic areas. Lentigo maligna and dermatofibrosarcoma protuberans accounted for a higher proportion of cases in our series, and repair of the surgical defect by a dermatologist was also more common. Operating times in MMS are not much longer than those reported for other procedures and the rate of intraoperative complications is very low.
El Registro Español de Cirugía de Mohs recoge los datos de aplicación y resultados de esta técnica en España. Se describen los datos de las intervenciones realizadas desde el inicio del Registro en julio de 2013 a enero de 2016. Se analizan los datos de las cirugías tanto perioperatorios como intraoperatorios.
Material y métodosEstudio de cohortes prospectivo en el que participan 18 centros. Se recogen los datos de las intervenciones quirúrgicas como tipo de anestesia, técnica quirúrgica, ingreso hospitalario, número de estadios, manejo de factores de riesgo preoperatorios, tratamientos complementarios, tratamientos previos, tipo de tumor, tiempo empleado en la cirugía y complicaciones.
ResultadosSe analizan 1.796 intervenciones quirúrgicas. El tumor intervenido con más frecuencia es el carcinoma basocelular (85,96%), seguido del carcinoma epidermoide (6,18%), lentigo maligno (2,81%) y dermatofibrosarcoma protuberans (1,97%). El 66,9% de los tumores eran primarios, el 19,2% recurrentes y el 13,9% persistentes. El tratamiento previo más frecuente fue quirúrgico. La cirugía de Mohs se realizó con más frecuencia bajo anestesia local (86,7%) y de forma ambulatoria (71,8%). En el 89,5% de los casos se utilizó la técnica de Mohs en congelación. El número de etapas requerido para alcanzar márgenes libres de tumor fue una en 56,45% de los pacientes, 2 en 32,1%, 3 en 7,1%, 4 en 2,7% y 5 o más en 1,8%. El propio dermatólogo reconstruyó el defecto en el 98% de los pacientes y la técnica reconstructiva más utilizada fue el colgajo (47,2%). Solo el 1,62% de los pacientes presentó alguna complicación intraoperatoria y la mediana de la duración de la cirugía fue 75 (p25:60-p75:100).
ConclusiónLas características de los pacientes y tumores tratados son similares a las descritas en estudios de las mismas características en otras áreas geográficas. Existe un porcentaje mayor de lentigo maligno y dermatofibrosarcoma protuberans. La reconstrucción la realiza el dermatólogo con más frecuencia que en otras series. El tiempo de utilización de quirófano no es mucho mayor que para otras técnicas y la tasa de complicaciones intraoperatorias es muy reducida.
Mohs micrographic surgery (MMS) is a surgical technique used to treat locally invasive malignant cutaneous tumors. It offers higher cure rates than other treatments and minimizes the removal of healthy peritumoral tissue.1
The original technique described by Dr. Mohs was the fixed-tissue technique, in which zinc chloride paste was applied to a lesion to achieve in situ fixation of the tumor tissue. The paste was removed after several hours and the tumor excised without anesthesia and with hardly any bleeding. The excised specimen was frozen and then horizontally sectioned for examination under a microscope.2 This procedure was repeated on successive days until all the margins of the final specimen were free of tumor. Dr. Mohs later described the fresh-tissue technique, in which successive layers of the tumor were removed under local anesthesia, without prior application of zinc chloride. This technique allowed surgeons to remove several layers of tumor and even repair the surgical defect on the same day and it became the method of choice for practically all MMS surgeons.3,4
The use of paraffin sections instead of frozen sections emerged for several reasons, including the difficulty of examining frozen sections for certain tumors, such as lentigo maligna (LM) and dermatofibrosarcoma protuberans (DFSP), the possibility of using immunostains in other cases, or simply lack of access to a cryostat. This modified procedure is known as slow Mohs. It has several variations, which are more common in Europe, that go under the names of 3D-histology, the perimeter technique, the Mohs-Tübingen technique, and the quadrant method. In all cases, however, including frozen-section MMS, 100% of tumor margins are examined through tangential sectioning rather than the standard vertical sectioning used in conventional surgery.5
Dr. Mohs’ technique was adopted first in the United States and then in Europe. An increasing number of Spanish hospitals from both the private and public health sectors have introduced MMS in recent decades, and their experience has been reported in both national and international journals.6–11 In 2012, the Spanish Academy of Dermatology and Venereology Foundation (FAEDV), in conjunction with the working group on Surgical Dermatology, Laser, and Cutaneous Oncology of the AEDV, decided to set up the Spanish Mohs Surgery Registry (REGESMOHS) in order to be able to describe the outcomes of MMS in Spain. The first cases were registered in July 2003. Previous studies have described the hospitals that perform MMS in Spain, together with the characteristics of the patients and tumors included in REGESMOHS.12,13
In this article we analyze the cases of MMS registered in REGESMOHS to date, with a focus on perioperative aspects (e.g., use of anticoagulants and antiplatelet agents), surgical technique, number of stages required to achieve tumor-free margins, surgical defect size and reconstruction, intraoperative complications, reconstruction of surgical defects, and use of resources.
Material and MethodsREGESMOHS is a prospective cohort study involving 18 public and private hospitals throughout Spain that perform at least 1 MMS operation a week. All patients who undergo evaluation prior to MMS are successively included in the registry. The only patients not included are minors and those declared to be legally incapacitated.
The study was approved by the research ethics committee of Navarra, the Spanish Agency for Medicines and Medical Devices (AEMPS), and all the participating hospitals. It was conducted in compliance with the principles of the Declaration of Helsinki and current laws. All patients gave their written consent to participate in the study.
The different outcome variables are collected during patient visits. Data on demographics, associated conditions such as immunosuppression and diabetes mellitus, clinical and histopathologic features of the tumor, and previous treatments are collected at the first visit. At the second visit, a record is made of type of surgery, anesthesia, hospitalization, use of anticoagulants and antiplatelet agents during surgery, surgical defect size, other treatments, surgery duration, and intraoperative complications and morbidity. Short-term outcomes and complications are evaluated at the third visit. Finally, patients are evaluated for surgical outcomes, recurrences, and appearance of new tumors at subsequent follow-up visits, which are held at least once a year. The development of subsequent tumors in a given patient is described in the registry but only the first tumor is subject to follow-up.
Data are entered by the participating hospitals into an online system provided by the Research Unit of the FAEDV (OpenClinica Open Source software, version 3.1) following a standard protocol. The information entered is monitored continuously online. Additional site checks are made once a year to assess adherence to the protocol and check the information entered against the source documents. Stata statistical software (version 14.1) is used for data analysis.
ResultsPatient CharacteristicsREGESMOHS contains data for 1796 patients who underwent at least 1 MMS operation at 1 of the 18 participating hospitals and whose information was added to the registry between July 2013 and January 2016 (Table 1). The characteristics of the population are similar to those previously described,13 although the size of the database has grown.
Number of Patients From Each Participating Hospital and Percentage of Total.
| Hospital | No. (%) of Patients |
|---|---|
| Hospital Santa Creu i Sant Pau | 345 (19.2) |
| Fundación Hospital Alcorcón | 235 (13.1) |
| Complejo Hospital Universitario de Guadalajara | 214 (11.9) |
| Complejo Hospitalario de León | 197 (11) |
| Clínica Universidad de Navarra | 109 (6.1) |
| Clínica Teknon | 108 (6) |
| Instituto Valenciano de Oncología | 84 (4.7) |
| Clínica Zarzuela | 82 (4.6) |
| Clínica Quirón Madrid | 78 (4.3) |
| Hospital del Mar | 69 (3.8) |
| Hospital La Princesa | 68 (3.8) |
| Hospital Galdakao | 60 (3.3) |
| Hospital de Cruces | 56 (3.1) |
| Hospital de Manises | 41 (2.3) |
| Hospital Gregorio Marañón | 26 (1.5) |
| Complexo Hospitalario Universitario de Santiago | 19 (1.1) |
| Hospital San Roque, Las Palmas | 4 (0.2) |
| Hospital La Paz, Madrid | 1 (0.1) |
| Total | 1.796 |
The patients’ characteristics are summarized in Table 2. The mean (SD) age of the sample was 68.5 (14.4) years, and 51.2% were men. In all, 77.7% of the population (n=1391) lived in the district served by the hospital where they underwent surgery. The remaining 22.4% (n=405) were referred from other districts. A history of multiple cutaneous tumors was recorded for 3.8% of patients (n=68), 6 of whom had a diagnosis of Gorlin syndrome. Sixty-eight patients (3.8%) had some form of immunosuppression, and the most common cause was solid organ transplantation (n=38, 2.1%). Finally, 10.2% of patients (n=179) were diabetic.
Patient Characteristics.
| Demographic Characteristics | No. (%) of Patients |
| Sex | |
| Male | 919 (51.2) |
| Female | 876 (48.8) |
| Place of residence | |
| Same health district as treating hospital | 405 (22.3) |
| Other health district | 1391 (77.7) |
| Mean (SD) age, y | 68.5 (14.4) |
| Clinical characteristics prior to surgery | No. (%) of Patients |
| Immunosuppression | |
| Transplantation | 38 (2.1) |
| Immunosuppressive treatment (>3 mo) | 19 (1.1) |
| Hematologic tumor <5 y | 11 (0.6) |
| Human immunodeficiency virus positivity | 3 (0.2) |
| Multiple tumor syndromes | |
| History of multiple tumors without a diagnosis of Gorlin syndrome | 55 (3.1) |
| Gorlin syndrome | 6 (0.3) |
| Other syndromes | 7 (0.4) |
| Diabetes mellitus | 179 (10.2) |
There were no relevant changes to the characteristics of tumors or to previous treatments with respect to the initial REGESMOHS report.13 Similar findings were also observed for tumor type (Table 3) and location. The most common locations, accounting for 82% of all tumor sites, were the nasal pyramid, the cheek, the forehead, the lateral canthus, and the auricular area. The median size of the largest tumor diameter was 10mm (interquartile range [IQR], 12mm). Just 1 patient had lymph node involvement and 2 had metastatic disease. Median time to inclusion of the tumor in the registry at the first visit was 11.4 months (IQR, 23.4 months), and median time from this point to surgery was 1.6 months (IQR, 1.9 months).
Frequency and Percentage Distribution by Histologic Type and Subtype.
| Histologic Type/Subtype | No. | % |
|---|---|---|
| Basal cell carcinoma | 1544 | 85.96 |
| Infiltrative | 734 | 41.24 |
| Histologically nonaggressive | 429 | 24.10 |
| Morpheaform/sclerosing/fibrosing | 220 | 12.36 |
| Micronodular | 113 | 6.35 |
| Metatypical/keratinization | 43 | 2.42 |
| Perineural | 5 | 0.28 |
| Squamous cell carcinoma | 111 | 6.18 |
| Infiltrative | 35 | 1.97 |
| Histologically nonaggressive | 35 | 1.97 |
| Basosquamous (not including metatypical basal cell carcinoma) | 17 | 0.96 |
| Undifferentiated | 9 | 0.51 |
| Sclerosing | 4 | 0.22 |
| Perineural/perivascular infiltration | 3 | 0.17 |
| Spindle-cell | 2 | 0.11 |
| Clear-cell | 2 | 0.11 |
| Small-cell | 1 | 0.06 |
| Keratoacanthoma (centrofacial) | 1 | 0.06 |
| Breslow>2 | 1 | 0.06 |
| > Clark level IV | 1 | 0.06 |
| Lentigo maligna | 50 | 2.81 |
| Dermatofibrosarcoma protuberans | 35 | 1.97 |
| Microcystic adnexal carcinoma | 4 | 0.22 |
| Merkel cell tumor | 4 | 0.22 |
| Extramammary Paget disease | 4 | 0.22 |
| Atypical fibroxanthoma/malignant fibrous histiocytoma | 2 | 0.11 |
| Angiosarcoma | 2 | 0.11 |
| Acral lentiginous melanoma | 1 | 0.06 |
| Sebaceous carcinoma | 1 | 0.06 |
| Eccrine/mucinous carcinoma | 1 | 0.06 |
| Desmoplastic trichoepithelioma | 1 | 0.06 |
Two-thirds of the tumors (66.9%, n=1200) had not been previously treated, 19.2% (n=345) were recurrent and had been treated either medically or surgically, and 13.9% (n=250) were persistent tumors, i.e., residual tumors at the margins following previous excision or tumors that had not responded to medical treatment.
Details of previous treatments and time to inclusion in the registry are shown in Table 4. The number of surgical treatments received by patients with persistent or recurrent tumors ranged from 1 (n=358) to 15 (n=1). Hedgehog inhibitors were used as adjunct therapy prior to MMS in 3 giant tumors.
Previous Treatments of Recurrent Tumors and Time Since Treatment.
| Treatments Before Mohs Micrographic Surgery | No. (%) of Patients | Median (IQR) Time Since Previous Treatment median, mo |
|---|---|---|
| Direct closure | 441 (24.6) | 21.2 (4.4-60.2) |
| Surgery with plasty repair | 123 (6.9) | 14.1 (2.6-59.9) |
| Cryotherapy | 95 (5.3) | 19.1 (8.6-55.5) |
| Surgery with grafting | 60 (3.3) | 36.4 (9.5-81.9) |
| Curettage/electrocoagulation | 49 (2.7) | 19.6 (2.4-70.5) |
| MMS | 42 (2.3) | 33.6 (18.9-69) |
| Radiation therapy | 19 (1.1) | 55.8 (12.4-149.1) |
| Photodynamic therapy | 21 (1.2) | 12.8 (8-19.5) |
| Imiquimod | 50 (2.8) | 15.1 (8.8-42) |
| Fluoropyrimidines | 1 (0.1) | 197.7 |
| Hedgehog inhibitors | 3 (0.2) | 11.3 (8-25.2) |
Abbreviation: MMS, Mohs micrographic surgery.
Table 5 shows the figures for use of anticoagulants and antiplatelet agents and their withdrawal prior to surgery, comedication with corticosteroids, concurrent diabetes mellitus, and presurgical factors that can affect healing.
Presurgical Factors That Can Affect Healing.
| Perioperative risk factors | No. (%) of Patients |
|---|---|
| Antiplatelet treatment | 191 (10.7) |
| Withdrawal | |
| No | 133 (70) |
| Yes | 57 (30) |
| Anticoagulant treatment | 130 (7.3) |
| Withdrawal | |
| No | 46 (35.7) |
| Yes | 83 (64.3) |
| Corticosteroid treatment >3 mo | 21 (1.2) |
| Diabetes mellitus | 179 (10.2) |
Surgery was performed on an outpatient basis or with a hospital admission time of under 24hours in 71.8% of cases (n=1420); the remaining 21.9% of patients (n=376) required hospitalization.
Local anesthesia was used in 86.7% of patients (n=1554), local anesthesia with oral premedication in 3.7% (n=67), and sedation or general anesthesia in 9.5% (n=171).
The most common MMS technique was frozen-section Mohs (89.5%, n=1603), followed by slow Mohs (10%, n=180), and MMS with 3D-histology (0.5%, n=9). MMS with intraoperative immunohistochemical staining was performed in just 7 cases (0.4%).
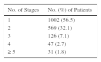
Presurgical curettage was performed at the discretion of the dermatologist. The stages required to achieve tumor-free margins are shown in Table 6. Tumor-free margins were not achieved in 56 patients (3.2%) for reasons related to the characteristics of the tumor, the poor general state of health of the patient, or patient preferences.
The median sizes (IQR) of the initial tumor and the final surgical defect are shown in Table 7. The tumor extended into the dermis in 61% of cases (n=1059), the hypodermis in 25.7% (n=445), and the muscle in 7.3% (n=127). Bone involvement was detected in 0.9% of cases (n=15).
Details of the specialist responsible for repairing the surgical defect are shown in Table 8.
No intraoperative complications were reported for the vast majority of patients (98.4%, n=1760). Twenty-nine patients experienced significant intraoperative morbidity, defined as a complication that required immediate intervention or modifications to the surgical procedure. The most common complication was vasovagal syncope (10 patients, 0.6%), followed by high blood pressure requiring treatment (8 patients, 0.5%) and bleeding requiring intravenous treatment or interruption of surgery (5 patients, 0.3%). There were also 2 episodes of hyperglycemia requiring treatment and unspecified arrhythmia in 1 patient (0.06%). All the complications were resolved immediately and surgery was completed in all cases but one.
Median duration of surgery was 75minutes (IQR, 40minutes; range, 15-438minutes).
Postoperative adjuvant therapy included radiation therapy (3 patients, 0.17%) and photodynamic therapy, imiquimod, radical surgery, and bone drilling and radiation therapy (1 case each).
DiscussionWe have described the current use of MMS in Spain based on information entered into REGESMOHS between July 2013 and January 2016. The data correspond to 1796 patients who underwent at least 1 MMS operation at one of the 18 participating hospitals. Our results provide insights into the use and technical complexity of MMS in Spain, as well as use of resources and short-term outcomes.
The strengths of the study lie in its prospective design and the analysis of data that are strongly representative of the state of MMS in Spain, as the participating hospitals account for a large proportion of all hospitals that perform this procedure in Spain.12
One limitation is related to the progressive inclusion of patients and recording of data, as some hospitals appear to have fewer patients than recorded in the initial report, indicating that they have either not operated on some patients or not entered the data into the online system. Minor changes to outcomes due to random variations can probably be expected in future publications.
The incidence of nonmelanoma skin cancer has increased exponentially in recent decades, and annual increases of between 3% and 8% have been reported in Europe, the USA, Canada, and Australia since 1960.14–16 MMS is the current gold standard for the treatment of high-risk BCC and SCC and it has also shown superior results to other techniques for other cutaneous cancers. A priori, however, the technique may not seem very cost-effective due to the infrastructure requirements, the need for trained personnel, and the time it takes. Data on these aspects of MMS can vary from country to country and accurate assessment is important for benchmarking purposes.
The use of MMS has been reported in several important studies, including prospective studies of a large Australian registry of patients treated with MMS between 1993 and 200217–19 and a series of prospective studies and randomized clinical trials comparing MMS and conventional surgery in the Netherlands.20–22
Our results for tumor location and patient sex are similar to those described in the initial report of REGESMOHS13 and other series. The only difference between our study and others that have included tumors other than BCC and SCC is the higher proportion of LM and DFSP cases. In our study, for example, these tumors accounted for 2.81% and 1.97% of cases, respectively, contrasting with rates of 0% and 0.5% in a recent study from the United Kingdom.22
Patients who had undergone solid organ transplantation represented the largest group of patients with immunosuppression in REGESMOHS (n=38). Transplant patients have a higher incidence of nonmelanoma skin cancer, and SCC in particular, and may be candidates for MMS.23,24
The proportion of patients who received antiplatelet or anticoagulant treatment (18%) is lower than that reported in other dermatologic surgery studies (29% for MMS patients25 and 35% for dermatologic surgery patients in general).26 The perioperative use of these treatments, however, was similar to that reported elsewhere.27,28
In our series, 33.1% of tumors were recurrent or persistent; this rate is identical to that reported for the Australian study of BCCs treated with MMS17 and very similar to the rate of 29.3% described by Hoorens et al.29 in the most recent study from the Netherlands. Like in our study, the most common previous treatment in these studies was surgery. It is noteworthy that, with the exception of cryotherapy, nonsurgical treatment was uncommon (< 3% for each treatment).
A mean of 1.6 stages (median, 1; range, 1-12) was required to achieve tumor-free margins. This figure is slightly lower than that of 1.7 (median, 2; range, 1-9) 17reported in Australia for BCC17 and identical to that of 1.6 (median, 1; range, 1-7) reported for SCC.19 In Europe, Flohil et al.20 reported a clearance rate of 43.5% with 1 stage (vs. 56.5% with ≥ 2 stages). In a series of 737 BCCs treated with MMS, Smeets et al.21 reported clearance rates of 25% and 46% for 1 and 2 stages, respectively. The corresponding rates for the UK series were 58% for 1 stage, 34% for 2 stages, 6% for 3 stages, 1% for 4 stages, and 0.5% for 5 or more stages.22
The surgical defect was repaired by a dermatologist in a very high proportion of patients in REGESMOHS (98.4% in total). On the whole, this rate is higher than previously reported rates. In a series of 12344 MMS operations performed in the United States (Brown University, Rhode Island) between 2005 and 2012, 17% of surgical defects in women and 8% of those in men were reconstructed by a plastic surgeon.30 In the Dutch series described by Hoorens et al.29 most of the surgical defects were repaired by dermatologists (84%), followed by head and neck surgeons (8.5%), plastic surgeons (6.2%), and other specialists, such as oculoplastic surgeons (1%-3%).
Flap closure, used in 47.2% of cases in our series, was the most common repair method used, coinciding with previous reports.31,32 Several decades ago, skin grafts used to be considered the most useful closure method in MMS, as they permitted earlier detection of possible recurrence. Now, however, flaps are more common than either grafting or healing by secondary intention due to the increased confidence in outcomes, improved surgical skills, smaller postsurgical defects, and the younger age of patients.
All the intraoperative complications reported in our series involved high blood pressure and/or intraoperative bleeding. Just 1 patient required hospitalization due to intraoperative- and postoperative bleeding, which is considered a major surgery-related complication.33,34
The median duration of surgery, 75minutes, indicates that, overall, MMS operations do not take much longer than conventional surgery of facial skin tumors that require reconstructive surgery with flap closure or grafts.
ConclusionsThe characteristics of the patients and tumors treated by MMS in Spain are similar to those reported in similar studies from other geographic areas. The findings for short-term outcomes were also similar. The main differences in our series were the higher percentage of DFSP and LM cases treated and the higher proportion of surgical defects repaired by a dermatologist. Major intraoperative complications were very rare and median duration of surgery was only slightly higher than that reported for conventional surgery. Our data are useful for evaluating whether findings from other settings can be applied to Spain for hospitals planning to introduce MMS or for those already using MMS wishing to use benchmarking to identify areas for improvement. Our results may also be useful for effectiveness studies and should interest dermatologists and other health care specialists and managers.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
FundingREGESMOHS is funded by FAEDV with the collaboration of Roche Farma. Roche Farma had no involvement in the preparation of this manuscript.
Conflicts of InterestThe authors declare that they have no conflicts of interest regarding the content of this article.
The following members of REGESMOH participated in the data collection stage of the study: Lucía Ascanio Armada and Dolores Caro Gutiérrez (Hospital Universitario Fundación Alcorcón); Carlos Serra Guillén, Eduardo Nagore Enguidanos, Beatriz Llombart Cussac, and Celia Requena Caballero (Instituto Valenciano de Oncología); Luis Hueso Gabriel and Antonio Martorell (Hospital de Manises); M. José Seoane Pose (CHU Santiago); Ricardo Suárez and Celia Horcajada (Hospital Gregorio Marañón); Francisco Alcántara Nicolás, Patricia González Muñoz, and Consuelo Sánchez Herreros (Hospital de Guadalajara); Carolina Medina Gil, Elena Castro González, and Jaime Vilar Alejo (Hospital San Roque, Las Palmas); María Luisa Alonso Pacheco and Matías Mayor Arenal (Hospital La Paz, Madrid). We also thank all the staff from the departments involved in REGESMOHS.
Please cite this article as: de Eusebio Murillo E, Martín Fuentes A, Ruiz-Salas V, Garcés JR, Miñano Medrano R, López-Estebaranz JL, et al. Descripción de las intervenciones quirúrgicas recogidas en el registro español de cirugía de Mohs (REGESMOHS) (2013-2015). Actas Dermosifiliogr. 2017;108:836–843.