Current psoriasis guidelines do not usually include recommendations about first line classical or biologic treatment. The objectives of this study were: to describe shifts in the prescription of the first biological treatment, and to compare treatment withdrawal and rates of adverse events over ten years.
Materials and methodsBiobadaderm registry was analyzed to describe: first biological prescription in bio-naïve patients, adverse events rate and reasons for drug withdrawal comparing three periods of time (2008-2010, 2011-2014, 2015-2018).
ResultsAntiTNF drugs were the most prescribed biological drug from 2008 to 2010. Ustekinumab has become the most prescribed first biologic since 2014. The main reasons for drug discontinuation were adverse events, lack of efficacy and remission. In each period any treatment was less likely to be discontinued due to any of these three reasons comparing to the previous period.
ConclusionsThe present study identifies trends in prescription of the first biological antipsoriatic drug in clinical practice from 2008 to 2018. It suggests that we have become more comfortable with the safety profile and more exigent with the efficacy of the drugs.
Las guías sobre el tratamiento de la psoriasis habitualmente no incluyen las recomendaciones acerca de cuál debe ser la primera línea de tratamiento sistémico o biológico. Los objetivos de este estudio fueron describir las tendencias en la prescripción del primer fármaco biológico y comparar la retirada de los fármacos y las tasas de efectos adversos a lo largo de los 10 años de seguimiento.
Material y métodosSe utilizó el registro Biobadaderm para determinar cuál fue el primer fármaco biológico indicado en pacientes con psoriasis “Naive” para biológicos, así como cuál es la tasa de efectos adversos y los motivos de suspensión de los fármacos. Los resultados obtenidos se compararon en tres periodos distintos de tiempo (2008-2010, 2011-2014, 2015-2018).
ResultadosLos fármacos anti-TNF fueron los biológicos prescritos con mayor frecuencia entre el año 2008 y el 2010. El Ustekinumab se ha convirtió en el tratamiento biológico más indicado a partir del 2014. El motivo principal de suspensión de los tratamientos fueron los efectos adversos, la falta de eficacia y la remisión de la enfermedad. La probabilidad de suspender los fármacos por uno de estos motivos fue cada vez menor si se compara con el periodo de tiempo previo.
ConclusionesEl presente estudio identifica cuáles fueron las tendencias en la prescripción del primer fármaco biológico en la práctica clínica habitual entre el año 2008 al 2018. Sugiere que los dermatólogos estamos cada vez más seguros en cuanto al perfil de seguridad y somos cada vez más exigentes en cuanto a la eficacia de los fármacos.
The availability of new biologics and molecules keeps changing the management of patients with moderate to severe psoriasis. A recent meta-analysis reviewed the best choices for achieving Psoriasis Area and Severity Index (PASI) 90 and found no differences among their security profiles.1 However, the choice of the first-line treatment for moderate to severe psoriasis in real life is not solely based on efficacy or security data.
In Spain, biologic drugs and apremilast are reimbursed by the National Health Service in patients with an inadequate response or contraindications to classical systemic therapy. Spanish and European guidelines do not include recommendations about first-line biologic treatments.2,3 However, recently updated British and French clinical guidelines have proposed ustekinumab or adalimumab as the first choice.4,5
The objectives of this study were to 1) describe shifts in the prescription of the first biologic or new small molecules treatment of psoriasis and 2) compare treatment withdrawal rates and the incidence of adverse events over ten years.
Patients and MethodsFor this study, we used the Biobadaderm registry, a prospective multicentre cohort registry of patients with psoriasis who were treated with systemic drugs aimed to detect adverse events related to systemic therapy. The data collection form and codification of the registry are described in a previous publication.6
To describe trends in treatment patterns over time, data were split in three quasi-equal time windows: (i) 2008–2010, (ii) 2011–2014, and (iii) 2015–2018. Only monotherapy and the first treatment in bio-naïve patients were considered for this analysis, as these patients reflect the first line therapeutic option and make the groups comparable.
Results were presented in two cohorts: biological or new small molecules (apremilast) therapy and classical therapy.
Characteristics of patients for each time group were presented using descriptive statistics according to the distribution of the variables: absolute numbers and percentages in qualitative data and the median and interquartile range in asymmetric variables. Differences between groups were compared using the Chi square test to determine the trend or the Wilcoxon–Mann–Whitney test.
Competing risks models were used to compare the cumulative incidence functions of discontinuation (due to inefficacy, adverse event or remission). We considered all other reasons for discontinuation (lost to follow-up, patient’s decisions, planned or desired pregnancy, etc.) as right censoring. We also described incidence rates of adverse events per 1,000 patient-years of exposure with 95% confidence intervals (CI) by period.
Data were analysed using Stata Statistical Software, Release 14.2 (StataCorp. 2015. College Station, TX: StataCorp LP), and all p-values less than 0.05 were considered statistically significant.
BIOBADADERM was approved by the Hospital 12 de Octubre Ethics Committee (216/07) and performed in compliance with the Declaration of Helsinki. All patients gave their written consent to participate.
ResultsIn total, 3,090 patients started a psoriatic therapy from 2008 until 2018, and 2,526 complied with the inclusion criteria (monotherapy and first drug in bio-naïve patients): 1,075 (43%) in the biological or new small molecules group and 1,451 (57%) in the classical group. Of the 2,526 patients, 43.4% (1,096) started their treatment from 2008 to 2010 (period 1), 29.4% (743) from 2011 to 2014 (period 2), and 27.2% (687) from 2015 to 2018 (period 3).
The demographic information for the surveyed population is summarized in Table 1. The following baseline characteristics of patients were different among the three periods over time in the classical group: different clinical psoriasis forms (more palmoplantar pustular psoriasis and less plaque and psoriatic arthritis), fewer comorbidities (hypertension, diabetes, and hypercholesterolemia), and lower age or disease duration in later periods. On the other hand, the biological or new small molecules group contained more females with hypertension, cancer, chronic liver disease, and chronic obstructive pulmonary disease, but with lower age, disease duration, and PASI.
Descriptive characteristics of bio-naïve patients from 2008 to 2018.
| Classical therapies | Biological therapies | |||||||
|---|---|---|---|---|---|---|---|---|
| 2008–2010 | 2011–2014 | 2015–2018 | P-value Trend | 2008–2010 | 2011–2014 | 2015–2018 | P-value Trend | |
| Total, N | 515 | 553 | 383 | 581 | 190 | 304 | ||
| Age, median (p25-p75) | 54.3 (44.5-68) | 50.7 (40.4-62) | 49.3 (38.3-58.8) | 0.0000*** | 53.3 (44.1-64.2) | 48 (39.9-59.2) | 51.7 (40.3-60.3) | 0.0001*** |
| Disease duration, median (p25-p75) | 12.1 (4.9-22.7) | 9.4 (3.4-19.7) | 9.3 (2.5-20.8) | 0.0005*** | 16.9 (9.3-27) | 13.6 (6.7-22.9) | 13.9 (6.5-26.2) | 0.0111* |
| Female, n(%) | 227 (44) | 232 (42) | 175 (46) | 0.7006 | 222 (38) | 74 (39) | 140 (46) | 0.0303* |
| Plaque psoriasis, n(%) | 468 (91) | 490 (89) | 327 (85) | 0.0110* | 562 (97) | 184 (97) | 271 (89) | 0.0000*** |
| Guttate psoriasis, n(%) | 30 (6) | 31 (6) | 22 (6) | 0.9482 | 24 (4) | 11 (6) | 10 (3) | 0.6738 |
| Erythrodermic psoriasis, n(%) | 11 (2) | 10 (2) | 2 (1) | 0.0632 | 10 (2) | 5 (3) | 5 (2) | 0.9682 |
| Generalized pustular psoriasis, n(%) | 10 (2) | 2 (0) | 2 (1) | 0.0208* | 4 (1) | 0 (0) | 4 (1) | 0.4004 |
| Palmoplantar pustular psoriasis, n(%) | 32 (6) | 37 (7) | 36 (9) | 0.0789 | 7 (1) | 5 (3) | 26 (9) | 0.0000*** |
| Annular pustular psoriasis, n(%) | 1 (0) | 1 (0) | 0 (0) | 0.4588 | 2 (0) | 0 (0) | 1 (0) | 0.8801 |
| Acrodermatitis continua of Hallopeau, n(%) | 1 (0) | 0 (0) | 0 (0) | 0.2445 | 0 (0) | 0 (0) | 2 (1) | 0.0407* |
| Psoriatic arthritis, n(%) | 52 (10) | 27 (5) | 15 (4) | 0.0001*** | 112 (19) | 21 (11) | 43 (14) | 0.0250* |
| ischemic heart disease, n(%) | 19 (4) | 12 (2) | 6 (2) | 0.0920 | 21 (4) | 3 (2) | 11 (4) | 0.9859 |
| Heart failure, n(%) | 7 (1) | 3 (1) | 6 (2) | 0.6697 | 6 (1) | 0 (0) | 5 (2) | 0.4379 |
| Hypertension, n(%) | 130 (26) | 108 (20) | 58 (18) | 0.0056** | 116 (20) | 35 (19) | 75 (27) | 0.0448* |
| Diabetes, n(%) | 65 (13) | 45 (8) | 19 (6) | 0.0008*** | 74 (13) | 18 (10) | 40 (15) | 0.6540 |
| Hypercholesterolemia, n(%) | 149 (30) | 136 (25) | 64 (20) | 0.0022** | 145 (26) | 44 (24) | 86 (31) | 0.1152 |
| Cancer, n(%) | 26 (5) | 23 (4) | 14 (5) | 0.6067 | 13 (2) | 2 (1) | 14 (5) | 0.0367* |
| Chronic liver disease, n(%) | 23 (5) | 8 (1) | 8 (3) | 0.0453* | 39 (7) | 10 (5) | 39 (15) | 0.0008*** |
| Renal insufficiency, n(%) | 7 (1) | 1 (0) | 4 (1) | 0.6251 | 11 (2) | 4 (2) | 6 (2) | 0.7772 |
| COPD, n(%) | 10 (2) | 15 (3) | 6 (2) | 0.9365 | 17 (3) | 4 (2) | 19 (7) | 0.0109 |
| Hepatitis B virus, n(%) | 23 (8) | 13 (4) | 13 (5) | 0.1637 | 25 (5) | 6 (3) | 19 (7) | 0.2580 |
| Hepatitis C virus, n(%) | 7 (2) | 9 (3) | 6 (2) | 0.9792 | 13 (2) | 6 (3) | 8 (3) | 0.6554 |
| HIV, n(%) | 5 (2) | 4 (1) | 2 (1) | 0.2505 | 0 (0) | 4 (2) | 0 (0) | 0.6192 |
| PASI, median (p25-p75) | 8.4 (4.3-12.1) | 9.4 (5.4-14) | 8 (5-12.5) | 0.9928 | 14 (9.6-20) | 12.6 (9.3-18) | 10 (6.4-14.4) | 0.0000*** |
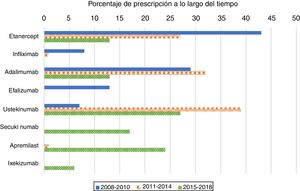
As expected, antiTNF drugs were the most prescribed (80%) first biologic treatment in bio-naïve patients during the 2008–2010 period (Figure 1). Its use as the first treatment dramatically declined during the second and third periods, representing 60% and 26% of prescriptions, respectively. When analysing antiTNF drugs separately, etanercept was the most prescribed first biologic in the first period, far more than the second, adalimumab. Starting in 2011, however, both etanercept and adalimumab became prescribed at a similar rate. Infliximab’s choice as the first biologic drug has been exceptional after 2010.
The use of ustekinumab varied significantly over time. It increased significantly in the second period and decreased with the appearance of new drugs after 2015.
Secukinumab, ixekizumab, and apremilast were prescribed for the first time in the 2015–2018 period and represented almost half of the treatments (47%); each were prescribed at higher percentages than those of etanercept and adalimumab, but lower than that of ustekinumab.
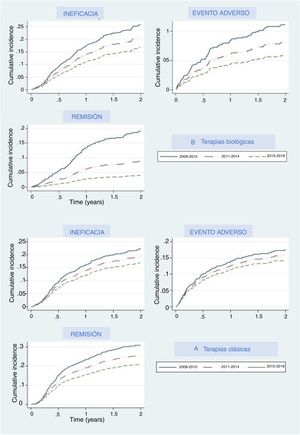
The main reasons for drug withdrawal were adverse events, lack of efficacy, and remission. Figure 2A represents the cumulative percentage of patients that discontinued their first treatment and what reason they give in the biological and new small molecules therapy group, and Fig. 2B shows this for the classical group. In both groups, cumulative incidence curves showed that, in recent years, participants were less likely to discontinue their treatment due to lack of efficacy, adverse events, or efficacy. In each period the thresholds were higher than the last, which means that treatments were less likely than in the previous period to be discontinued for any of these three reasons, especially remission, where curves were the most separated. Regarding biological and apremilast therapy, discontinuation due to remission showed a drastic increase in the threshold (decrease in the curve) in the last period (2015–2018), which means that fewer patients withdrew due to remission, as the cut-off point to consider a patient in remission increased. A similar, although less pronounced, pattern could be seen concerning adverse events.
The rates of all adverse events statistically went down over the years, while the rates of serious or fatal adverse events remained stable over time (Table 2). Regarding the specific rates per treatment in the biological and apremilast group, it seems that the new therapies (apremilast and ixekizumab) have higher rates compared to ‘older’ ones (anti-TNF and ustekinumab). These rates are comparable to those for antiTNF and ustekinumab during the first period.
Adverse events occurred in bio-naïve patients from 2008 to 2018.
| Biologic therapies | Classical therapies | |||||
|---|---|---|---|---|---|---|
| 2008–2010 | 2011–2014 | 2015–2018 | 2008–2010 | 2011–2014 | 2015–2018 | |
| All adverse events, n (patient-years) | 920 (1468) | 280 (581) | 157 (352) | 734 (705) | 686 (787) | 172 (360) |
| Serious adverse events, n (patient-years) | 79 (1468) | 26 (581) | 9 (352) | 58 (705) | 49 (787) | 20 (360) |
| Non-Serious adverse events, n (patient-years) | 833 (1468) | 254 (581) | 146 (352) | 664 (705) | 633 (787) | 151 (360) |
| Fatal adverse events, n (patient-years) | 8 (1468) | 0 (581) | 2 (352) | 12 (705) | 4 (787) | 1 (360) |
| Incidence rate (per 1000 person-year) (CI95%) | ||||||
| All adverse events | 627 (588-669) | 482 (429-542) | 446 (381-522) | 1041 (969-1120) | 872 (809-939) | 478 (412-555) |
| Serious adverse events | 54 (43-67) | 45 (30-66) | 26 (13-49) | 82 (64-106) | 62 (47-82) | 56 (36-86) |
| Non-Serious adverse events | 568 (530-607) | 437 (387-494) | 415 (353-488) | 942 (873-1017) | 804 (744-869) | 420 (358-492) |
| Fatal adverse events | 5 (3-11) | 0 (-) | 6 (1-23) | 17 (10-30) | 5 (2-14) | 3 (0-20) |
| All adverse events per treatment, rate (per 1000 person-year) (CI95%) | ||||||
| Etanercept | 625 (563-694) | 549 (431-699) | 270 (157-465) | |||
| Infliximab | 503 (414-611) | 994 (414-2388) | 0 (-) | |||
| Adalimumab | 655 (590-728) | 616 (506-750) | 420 (288-613) | |||
| Ustekinumab | 557 (453-686) | 155 (22-1099) | 377 (277-514) | |||
| Secukinumab | – | – | 464 (322-667) | |||
| Apremilast | – | – | 705 (519-958) | |||
| Ixekizumab | – | – | 650 (310-1364) | |||
| Acitretin | – | – | – | 920 (809-1046) | 871 (749-1013) | 343 (250-469) |
| Cyclosporine | – | – | – | 1732 (1482-2025) | 1858 (1575-2191) | 823 (564-1200) |
| Methotrexate | – | – | – | 952 (857-1058) | 727 (657-804) | 497 (411-601) |
This population-based drug utilization study evaluated the shift in the number of prescriptions of the first biological drug and apremilast over ten years in a real-world setting among patients diagnosed with psoriasis. There was a trend towards prescribing more etanercept during the 2008–2010 period, much more than adalimumab. However, probably owing to the introduction of ustekinumab in 2009, the use of both etanercept and adalimumab decreased significantly and have showed similar and stable rates of prescription since then. In the US, etanercept was initially the most preferred medication, but adalimumab use increased gradually after 2011 following its approval for psoriatic arthritis in 2005 and for psoriasis in 2008.7 According to our registry, adalimumab followed a different trend, with a clear decrease after 2011. Also, ustekinumab became the most prescribed first biologic since 2014. A recent metanalysis concluded that current evidence suggests that ustekinumab is the preferred biologic for treating psoriasis.8 Moreover, British and French association guidelines recommend ustekinumab or adalimumab as the first biologic treatment in psoriatic patients.4,5
IL-17 blockers have very high efficacies and are likely to compete with ‘older’ biologics, thus changing the prescription trends. During the third period (2015–2018), secukinumab prescription exceeded antiTNF as the first biologic drug in bio-naïve patients and almost equalled ustekinumab.
Apremilast has shown a lower efficacy compared to biologic drugs,9,10 however, its use as a first treatment is comparable to that of secukinumab during the 2015–2018 period. The safety profile of apremilast,11 in addition to its lower cost compared to biologics, probably played an important role in its selection. Some experts, however, do not recommend that the drug be used on its own. For example, French guidelines recommend biological agents prior to initiating systemic treatment with apremilast.4
Even though more effective drugs became available after 2011, both etanercept and adalimumab were the first prescribed drugs in 6–8% of the patients. This may be due to the presence of psoriatic arthritis and to the fact that many dermatologists may feel more comfortable using the ‘older’ drugs.
In our study, the major reason for treatment termination was loss of efficacy. This has been previously described in our registry12 and in other large studies.13,14
Our analysis allowed us to compare different time periods, and this comparison showed that the discontinuation rate decreased over time, and fewer patients discontinued biological agents or apremilast due to lack or loss of efficacy or to AE. Dermatologists seem to be more tolerant of non-serious adverse events, leading to both lower notification and discontinuation rates. This is probably due to the increasing knowledge on the security profile of these molecules. We also recently demonstrated that the overall rates of adverse events were higher during the first year of treatment with all drugs.15 Severe adverse event rate, however, remained stable over time, which implies that the patients were under constant surveillance over time.
Interestingly, remission is harder to achieve nowadays compared to previous periods, as that discontinuation rates decrease over time. This may be due, in part, to patient dissatisfaction following minor disease relapse. A shift in patient expectations to achieve PASI 90–100 has become more common, driving lower rates of discontinuation due to remission, in real-world clinical practice.16
In conclusion, the present study identifies behaviours and trends in prescription rates of the first biological antipsoriatic drug and new small molecules (apremilast) in clinical practice from 2008 to 2018 in Spain. It also suggests that dermatologists are managing their patients differently and becoming more comfortable with drug safety profiles and more exigent with drug efficacies, leading to lower discontinuation rates.
FinancingThe BIOBADADERM project is promoted by the Fundación Piel Sana Academia Española de Dermatología y Venereología, which receives financial support from the Spanish Medicines and Health Products Agency (Agencia Española de Medicamentos y Productos Sanitarios) and from pharmaceutical companies (Abbott/Abbvie, Pfizer, MSD, Novartis, Lilly, Janssen and Almirall).
Collaborating pharmaceutical companies were not involved in the: design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; decision to submit the manuscript for publication.
Conflict of interestDr Ruiz-Genao has been reimbursed by Pfizer, Janssen, Celgene, Abbvie, Novartis and LeoPharma for advisory services and conferences.
Dr Carretero has been reimbursed by Janssen, Abbvie, Novartis, Pfizer, MSD and Celgene for advisory service and conference.
Dr Rivera acted as consultant and/or speaker for and/or participated in clinical trials as IP for Abbvie, Almirall, Celgene, Janssen, Leo Pharma, Lilly, Novartis, MSD and Pfizer-Wyeth.
Dr Ferrándiz has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Merck Sharp & Dohme, Novartis Pfizer and Almirall.
Dr Dauden acted as consultant for Abbott, Amgen, Astellas, Centocor Ortho Biotech Inc, Galderma, Glaxo, Jansenn-Cilag, Leo Pharma, Novartis, Pfizer, MSD and Celgene, received honoraria form Abbott, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD, Celgene, participated in a speakers bureau for Abbott, Pfizer, MSD and Janssen and received grants from Pfizer, Abbott, Janssen and MSD.
Dr. De la Cueva acted as a consultant and/or speaker for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, UCB, Biogen, Celgene, Amgen, Sandoz, Sanofi and Leo-Pharma.
Dr Belinchón acted as a speaker and/or advisor for Janssen Pharmaceuticals Inc, Almirall SA, Lilly, AbbVie, Novartis, Celgene, Biogen Amgen, Leo-Pharma, Pfizer-Wyeth, and MSD.
Dr Herrera-Acosta has served as consultant and/or speaker with Leo Pharma, Novartis, Janssen, Lilly, Celgene y Abbvie.
Dr López-Estebaranz participated as AB and received educational grants from Janssen, Abbvie, MSD, Lilly, Novartis, LeoPharma, Pfizer.
Dr Ferrán-Farrés has participated as speaker and/or advisor for Janssen, Lilly, Novartis, Pfizer, MSD, Abbvie Celgene and Almirall.
Dr Alsina gave expert testimony for Merck-Schering Plough, Pfizer, Janssen, Novartis and Abbott.
Dr Baniandrés-Rodríguez acted as a consultant and/or speaker for Janssen-Cilag, AbbVie, Pfizer, Novartis, Lilly, Celgene, Leo Pharma and Almirall.
Dr Sánchez-Carazo participated as AB from Janssen, Novartis and Leo Pharma.
Dr Sahuquillo has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer.
Dr Rodriguez Fernandez-Freire acted as a consultant and speaker for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, Celgene and Leo-Pharma.
Dr Vilar-Alejo participated as AB from Janssen, Novartis, AbbVie, Almirall and Celgene.
Dr García-Donoso participated as AB from AbbVie, Almirall and speaker for Janssen, Lilly and Celgene.
Dr Carrascosa has participated as speaker and/or advisor for Celgene, Janssen, Lilly, Novartis, Leo Pharma, Pfizer, MSD, Abbvie, Biogen Amgen.
Dr Llamas acted as a consultant and speaker for Janssen-Cilag, AbbVie, Celgene, Pfizer, Novartis, Lilly, Almirall and Leo-Pharma and has participated in clinical assays.
Dr. Herrera-Ceballos has served as a consultant and/or speaker for and/or participated in clinical trials as IP and sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer.
Dr Botella has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer.
Dr Garcia-Doval received travel grants for congresses from Abbvie, MSD and Pfizer.
None of the other authors has any conflicting interests to declare.
IRB status:
Observational study. Approved (Biobadaderm: Hospital Universitario 12 de Octubre (216/07).
AnnexThis work was conducted within the BIOBADADERM Study Group. The following members participated in acquisition of data and review of the manuscript: Esteban Daudén, Mar Llamas-Velasco (Hospital Universitario de la Princesa); Gregorio Carretero, Jaime Vilar-Alejo (Hospital Universitario de Gran Canaria Dr. Negrín); Raquel Rivera, Carmen García-Donoso (Hospital Universitario 12 de Octubre); Carlos Ferrándiz, José Manuel Carrascosa, Ferrán Ballescá (Hospital Universitari Germans Trias i Pujol); Pablo de la Cueva (Hospital Universitario Infanta Leonor); Isabel Belinchón (Hospital General Universitario de Alicante); Fran J. Gómez-García, Rafael Jiménez (Hospital Universitario Reina Sofía); Enrique Herrera-Ceballos, Enrique Herrera-Acosta (Hospital Universitario Virgen de la Victoria); José Luis López-Estebaranz, Diana Patricia Ruiz-Genao (Fundación Hospital de Alcorcón); Marta Ferrán Farrés (Hospital del Mar, Parc de Salut Mar de Barcelona); Mercè Alsina (Hospital Clinic de Barcelona); Ofelia Baniandrés, Lula Nieto (Hospital General Universitario Gregorio Marañón); José Luis Sánchez-Carazo (Hospital General Universitario de Valencia); Antonio Sahuquillo-Torralba, Rafael Botella-Estrada, Conrad Pujol Marco (Hospital Universitario La Fe de Valencia); Lourdes Rodríguez Fernández-Freire (Hospital Universitario Virgen del Rocío de Sevilla); Almudena Mateu Puchades (Hospital Universitario Dr. Peset), Ángeles Flórez Menéndez, Laura Salgado, Beatriz González Sixto (Complexo Hospitalario Universitario de Pontevedra); Noemí Eiris (Complejo Asistencial Universitario de León); Ignacio García-Doval, Miguel Ángel Descalzo Gallego, Marina de Vega Martínez (Fundación Piel Sana AEDV).
The other members of the BIOBADADERM group are mentioned in the Annex.
Please cite this article as: Ruiz-Genao DP, Carretero G, Rivera R, Ferrándiz C, Daudén E, de la Cuev P, et al. Cambios en las tendencias de la prescripción y causas de la interrupción en los tratamientos biológicos indicados en la psoriasis durante los primeros 10 años. Datos obtenidos del registro español Biobadaderm. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.05.008