Blisters associated with PUVA treatments are an adverse effect of photochemotherapy that has been reported in the literature. Asymptomatic blisters appear spontaneously mainly on the lower limbs and resolve without treatment. The differential diagnoses to consider include a phototoxic reaction, pseudoporphyria, and PUVA-induced bullous pemphigoid. We describe the clinical and histologic features in 5 cases of blistering secondary to PUVA treatment. If this adverse effect is accurately diagnosed, photochemotherapy need not be interrupted, and unnecessary diagnostic procedures and additional treatments can be avoided.
Las ampollas secundarias al tratamiento con PUVA son un efecto secundario de la fotoquimioterapia poco descrito en la literatura científica. Se caracteriza por la aparición espontánea de ampollas asintomáticas localizadas fundamentalmente en los miembros inferiores, que se resuelven sin necesidad de tratamiento. El diagnóstico diferencial debe plantearse con una reacción fototóxica, con la pseudoporfiria y con el penfigoide ampolloso inducido por PUVA. Presentamos 5 casos de ampollas secundarias a la terapia PUVA, con el objetivo de dar a conocer las características clínicas e histológicas de dicha entidad. Su correcto diagnóstico evitará la interrupción del tratamiento, así como la realización de procedimientos diagnósticos y terapéuticos innecesarios.
Photochemotherapy is a first-line therapy for skin conditions such as psoriasis and mycosis fungoides and an alternative treatment option for atopic dermatitis or vitiligo patients who are intolerant or refractory to first-line treatment.1,2 PUVA (psoralen and ultraviolet A [UVA]) treatment, administered as either oral or bath PUVA, is both safe and widely used but is associated with short- and long-term adverse effects. One of the known adverse effects of PUVA treatment is the formation of blisters, which can be spontaneous and self-limiting or can be secondary to a phototoxic reaction, PUVA-induced bullous pemphigoid, or pseudoporphyria.1,3
We describe the clinical and histological characteristics of self-limiting blisters that developed during PUVA treatment of 5 patients at the Phototherapy Unit of the University Hospital of Cabueñes. If this adverse effect is rapidly and accurately diagnosed, treatment interruption and unnecessary diagnostic procedures and additional treatments can be avoided.
Case DescriptionsThe clinical characteristics of the 5 patients, 3 men and 2 women (mean age, 65.8 y; range, 44–86 y), are shown in Table 1.
Clinical Characteristics of Patients With PUVA-Induced Acral Blisters.
| Patient | Sex | Age, y | Phototype | Personal History | Treatments | Dermatological Diagnosis | PUVA Modality | Number of Flare-ups | Location | Dose of Current Cycle (J/cm2) | Maximum Dose (J/cm2) | Dose at Which Blisters Appeared (J/cm2) | Previous Cumulative Dose (J/cm2) | Total cumulative dose (J/cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | II | HT | Indapamide, sertraline | Psoriasis | Bath PUVA | 1 | Both feet | 3.25 | 0.75 | 0.75 | 0 | 3.25 |
| 2 | M | 61 | IV | HT, DLD | Enalapril and HCTZ, sinvastatin | Mycosis fungoides | Oral PUVA | 1 | Right leg and foot | 90 | 10 | 10 | 0 | 90 |
| 3 | F | 65 | IV | HT, GER | Amlodipine, rabeprazole, enalapril and HCTZ | Plaque parapsoriasis | Bath PUVA | 3 | Left foot | 26.05 | 4 | 2.70 | 120.5 | 146.1 |
| 4 | M | 44 | III | Ichthyosis X, DA | Acitretin | Mycosis fungoides | Bath PUVA | 4 | Both legs and feet | 6.1 | 3.40 | 3.10 | 84.2 | 90.3 |
| 5 | M | 86 | III | COPD, BPH | Dutasteride and tamsulosin, doxepin, hydroxyzine, tiotropium bromide | Mycosis fungoides | Oral PUVA | 1 | Left leg | 4 | 1.50 | 0.75 | 0 | 4 |
Abbreviations: AD, atopic dermatitis; BPH, benign prostatic hypertrophy; COPD, chronic obstructive pulmonary disease; DLD, dyslipidemia; F, female; GER, gastroesophageal reflux; HCTZ, hydrochlorothiazide; HT, hypertension; M, male; PUVA, psoralen and ultraviolet A.
Three of the patients underwent bath PUVA (immersion for 15min in 2.4mg/L methoxsalen at 37–42°C) and the other 2 patients received oral PUVA (0.6mg/kg Oxsoralen 2h before exposure to UVA). The initial UVA dose was determined based on patient phototype and increments were calculated based on the previous dose received. Patients underwent 2 or 3 sessions per week. The maximum and cumulative doses, as well as the dose at which blisters appeared, are indicated in Table 1.
Treatment was administered using a UV7001K phototherapy cabinet (PUVA/UV21, Waldmann, Germany) equipped with 40 lamps, including 27 Waldmann F85/100 W-PUVA lamps (spectral range, 315–400nm; maximum emission, 355nm). All patients wore protective glasses during each session and male patients wore black underwear to protect the genital region.
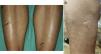
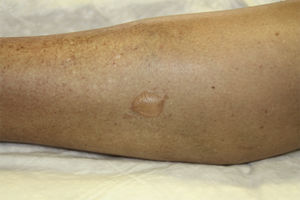
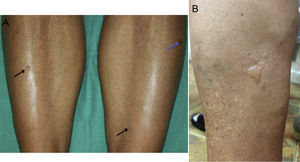
All patients developed small- or medium-sized blisters on the lower limbs that contained serous fluid (Figs. 1 and 2). In no case was it necessary to interrupt treatment or to adjust the doses of UVA or methoxsalen. As a protective measure, blisters were occluded before each session and treated daily with topical antiseptic until resolution. In all cases the blisters disappeared spontaneously after a mean duration of 7 days. No worsening of the underlying skin condition was observed in the temporarily occluded areas.
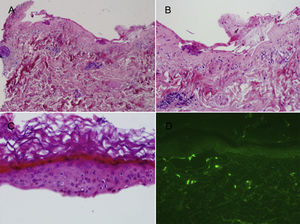
Samples for histopathology were acquired from 2 patients. Blister samples were collected for hematoxylin-eosin staining and healthy perilesional skin samples for analysis by direct immunofluorescence (DIF). In both patients, histopathology showed a subepidermal blister with fibrinoid deposits on the dermal floor and mild inflammatory infiltrate (Figs. 3A and 3B). Devitalization of the basal layer and keratinocyte necrosis were evident in the detached epidermis (Fig. 3C). The results of the DIF study were negative for both patients (Fig. 3D). Analyses requested for 1 patient, including tests for antinuclear antibodies and total extractable nuclear antibodies, revealed no noteworthy alterations.
A, Subepidermal blister with mild inflammatory infiltrate (hematoxylin-eosin, original magnification ×4). B, Fibrinoid deposits on the dermal floor with scarce inflammatory infiltrate (hematoxylin-eosin, original magnification ×10). C, Detached epidermis in which devitalization of the basal layer and necrotic keratinocytes are observed (hematoxylin-eosin, original magnification ×20). D, Negative direct immunofluorescence test.
Known adverse effects of PUVA treatment include phototoxic reactions, skin hyperpigmentation, an increased risk of carcinogenesis (mainly squamous cell carcinoma), and the appearance of signs of actinic damage, such as lentigines and actinic keratosis. However, to avoid unnecessary interruption of treatment it is important to be familiar with other, less common adverse reactions.
Up to 10% of patients who receive PUVA treatment develop blisters.7,8 Nonetheless, descriptions of this adverse effect are scarce,4–9 perhaps because these asymptomatic and self-limiting blisters go unnoticed.
Clinically, this condition is characterized by the presence of asymptomatic, small- or medium-sized nonhemorrhagic blisters on healthy, nonerythematous skin, mainly on the lower limbs.
While phototoxic reactions usually occur during the initial phase of treatment, the appearance of PUVA-induced blisters is predominantly associated with the dose administered during the last 30 days of treatment, not with the last dose administered nor the total dose.4,5 Heidbreder and Henseler reported a negative correlation between patient age and blistering time.5
The main histopathological findings described in psoriatic patients7,9 are subepidermal blisters with variable epidermal involvement, ranging from complete necrosis of the Malpighian layer to the presence of isolated necrotic keratinocytes. Dermal involvement tends to be mild, with isolated, predominantly lymphocytic perivascular infiltrate. The DIF test is usually negative, although the presence of deposits of complement component C3 in the perilesional skin has been described in some cases.8,9
The pathogenesis of this adverse reaction remains unclear. Several hypotheses have been proposed to explain the increased fragility of the dermis and epidermis. The variable numbers of necrotic keratinocytes revealed by histopathology suggest that blister formation may be the result of a phototoxic reaction,7,8 while the near exclusive location of the lesions on the lower limbs suggests that friction and trauma may trigger lesion formation.8 Heidbreder and Henseler5 demonstrated that PUVA treatment inhibits the synthesis of connective tissue and increases collagen fractionation, resulting in a loss of dermoepidermal cohesion. Finally, Friedmann and colleagues8 proposed that blister formation may be induced by complement component C3, deposits of which are found in the perilesional skin. However, this proposed role of complement in blister development is at odds with the absence of immunoglobulins and other immunoreactants and the paucity of inflammatory infiltrate.8
Three main conditions should be included in the differential diagnosis: blisters secondary to phototoxic reactions, pseudoporphyria, and PUVA-induced bullous pemphigoid.10 The main characteristics of each of these conditions are listed in Table 2.
Differential Diagnosis of PUVA-Induced Acral Blisters.
| PUVA-Induced Acral Blisters | Bullous Pemphigoid | Phototoxic Reaction | Pseudoporphyria | |
|---|---|---|---|---|
| Clinical | 10%7,8 | Unknown | 11%11 | Unknown |
| incidence | Small-to medium-sized asymptomatic serous blisters on healthy, nonerythematous skin | Tense, pruritic, serohemorrhagic blisters of various sizes on healthy or erythematous skin, erosions, scabs | Severe erythema, serous blisters on erythematous skin, intense pruritus | Tense symptomatic vesicles and blisters; skin fragility; erosions; scars; milium cysts |
| Location | Acral regions (LL) | Any location | Large areas of skin (breasts or buttocks) | Photoexposed areas (backs of hands, face, extension surface of LL) |
| Histology | Subepidermal blisters, necrotic keratinocytes, mild dermal infiltrate | Subepidermal blister with eosinophils and PMNs, perivascular edema, dermal lymphohistiocytic infiltrate with eosinophils | Spongiosis, subepidermal edema with vesiculation and sunburn cells | Porphyria cutanea tarda (subepidermal blisters in which the festoons of dermal papillae are preserved, mild perivascular lymphocytic infiltrate) |
| DIF | Negative (in some cases, perilesional deposition of C3) | Positive (linear deposition of C3 and IgG along the basement membrane) | Negative | Positive (deposition of IgG, complement, and fibrinogen at the dermoepidermal junction and on the walls of small vessels) or negative |
| Management | Do not interrupt treatment Local antiseptic | Interrupt treatment Treatment of BP | Interrupt treatment Symptomatic treatment | Treat trigger Symptomatic treatment |
Abbreviations: BP, bullous pemphigoid; C3, complement component C3; DIF, direct immunofluorescence; IgG, immunoglobulin G; LL, lower limbs; PMN, polymorphonuclear neutrophil; PUVA, psoralen and ultraviolet A.
Phototoxic reactions have been reported in 11% of patients, and are more frequent in those with light skin phototypes (I and II).11 They are characterized by extensive pruritic erythema that generally reaches maximum intensity after 48 to 72hours and, unlike PUVA-induced acral blisters, necessitates interruption of treatment.3 The presence of sunburn cells in histology is characteristic. Phototoxic reactions can be caused by UVA overdose; methoxsalen overdose or toxicity, which is usually accompanied by gastrointestinal signs; concurrent administration of photosensitizing drugs, such as doxycycline or methotrexate; or failure by patients to take appropriate photoprotective measures.11
Pseudoporphyria is a rare, photodistributed bullous dermatosis characterized by clinical, histological, and DIF findings similar to those of porphyria cutanea tarda, but without alterations in porphyrin levels. Multiple factors, including chronic renal failure, dialysis, excessive sun exposure, PUVA treatment, and treatment with drugs such as NSAIDs, antibiotics, and diuretics are implicated in the development of pseudoporphyria.12,13
Several external factors, including PUVA treatment, have been associated with the development of bullous pemphigoid.14–16 PUVA-induced alterations in basement membrane proteins could induce an immune response mediated by autoantibodies that cross react with basement-membrane proteins. The lesions can appear on normal or erythematous skin, and even non-photoexposed areas. Histologically, bullous pemphigoid is characterized by subepidermal blisters with lymphohistiocytic infiltrate containing eosinophils, and DIF shows linear deposition of IgG and complement component C3 at the dermoepidermal junction.15,16 Bullous pemphigoid is considered a minor contraindication to PUVA treatment.17
The prognosis of patients with PUVA-induced blisters is excellent, given the self-limiting nature of the lesions. Topical antiseptic to avoid superinfection is the only treatment indicated. Because interruption of PUVA treatment is not necessary in these cases, it is important to be able to correctly identify this adverse effect.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vázquez-Osorio I, González-Delgado S, Suárez-García C, Gonzalvo-Rodríguez P, Rodríguez-Díaz E. Blisters Induced by PUVA: A Report of 5 Cases. Actas Dermosifiliogr. 2018;109:e11–e16.