Atopic dermatitis is a common chronic inflammatory cutaneous disease with a great impact on the quality of life. It affects nearly 10–30% of children and 2–10% of adults.1 Dupilumab was the first approved biologic therapy for this disease and is now approved for different age groups, including children as young as 6 years old with severe AD. It is a fully human monoclonal antibody that targets the alpha subunit of the interleukin 4 receptor (IL-4Rα), thus modulating the signaling of both IL-4 and IL-13. This recent drug is considered as being immunomodulatory and not immunosuppressant because of its mechanism of action. In fact, the clinical trials with dupilumab, showed no evidence of an increase in overall infection rates and evidence of no increased risk of serious or opportunistic infections in dupilumab-treated patients.2 Real world evidence of the safe use of dupilumab in atopic dermatitis patients with viral infections such as HIV and hepatitis B has emerged, but it is important to gather data in other infections such as hepatitis C infection.3–6 Despite the drug not being formally contraindicated for AD patients with HCV infection under anti-viral treatment, there's no data regarding the use of dupilumab in this special population from the clinical trials, because having an active chronic or acute infections including HIV and hepatitis B and C was an exclusion criteria.7 To date, no data has been published regarding the use of dupilumab in the treatment of atopic dermatitis patients with hepatitis C. The author reports a case of an atopic dermatitis patients with active hepatitis C, successfully and safely treated with dupilumab.
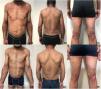
A 47-years-old male patient was observed in our consultation due to severe atopic dermatitis since the age of 32. He had a prior medical history of active hepatitis C and was being monitored in the Gastroenterology department. Clinically, the patient presented with extensive and lichenified lesions, with a BSA of around 60% and an EASI score of 40.9 (Fig. 1a–c). He reported a DLQI score of 27 and a pruritus NRS of 9. The patient started treatment with dupilumab, with significant overall improvement of the atopic dermatitis, reaching an EASI-90 response by month 6, with a DLQI score of 3 and pruritus NRS of 0 (Fig. 1d–f). During the treatment, there was no worsening of the hepatitis (no changes in liver enzymes or viral load), with the patient undergoing hepatitis C treatment with Sofosbuvir and Ledipasvir and achieving clinical remission (normalization of liver enzymes and viral load of 0).
In patients with systemic infections such as HIV/AIDS or hepatitis C, once topical treatments and phototherapy fail, systemic treatment options for these patients are extremely scarce. Dupilumab is an effective and safe treatment for atopic dermatitis. Due to the mechanism of action (inhibition of the T-helper 2 pathway), it preserves the ability of the immune system to effectively react to viral infections (through T-helper 1 cells), even decreasing the frequency of some such as herpetic infections. It is also worth to mention that some investigational works suggest that IL-4 is overexpressed in liver grafts in a context of severe recurrent hepatitis C, which may indicate that this interleukin has a potential role in the accelerated course of fibrogenesis during recurrent HCV infection.8 Studies have also shown that Th2 cytokines have a role in tissue fibrosis involving other organs such as the lung and the skin. Some data suggest that these profibrotic actions of IL-4 (and IL-13) occur via transforming growth factor-beta (TGF-β) pathway and periostin production and secretion.9 So theoretically, dupilumab may be safely used in patients with systemic viral infections such as hepatitis C as we’ve seen with hepatitis B infection cases, and may have a beneficial effect in patients with a degree of liver fibrosis. Most importantly, there's a recent publication by the International Eczema Council (IEC) that ranked dupilumab as first-line in the preferred systemic treatments for adults with AD who have a chronic hepatitis B and/or C viral infection.10
In this case, not only did the hepatitis not worsen, but the patient was able to undergo treatment and cure the infection while under treatment with dupilumab. To the best of our knowledge, this is the first reported case of an atopic dermatitis patient with active hepatitis C infection treated with dupilumab. Given the experience of this case and knowing the results of the IEC survey, in which dupilumab was selected as first-choice for these situations, the decision to start dupilumab seems to be clearer and more obvious today. However, more data is required regarding the use of dupilumab for the treatment of atopic dermatitis patients with systemic infections.
Conflict of interestF. Mota has received honoraria for acting as a consultant and/or as a speaker for Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer and Sanofi Genzyme.
He has also worked as a co-investigator in clinical trials supported by Amgen and Novartis.