Type I interferon (IFN-I) signaling plays a key role in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE). Anifrolumab – a monoclonal antibody targeting the interferon-alpha receptor subunit 1 (IFNAR1) – has been recently approved by the FDA and EMA for the treatment of SLE. In two phase III clinical trials, it has demonstrated efficacy in treating CLE in patients with SLE. Several isolated cases and small case series of CLE with a good response to anifrolumab have been published.
We conducted a retrospective observational study in four hospitals in the Valencian Community (Spain). Patients with CLE treated with IV anifrolumab (300mg/4 weeks) for, at least, 16 weeks were included. The endpoints were to determine the rates of partial (≥50% reduction in RCLASI-A) and complete responses (RCLASI-A 0), the mean reduction in RCLASI-A at 16 weeks, and the median time to achieve a partial response.
A total of 9 patients (Table 1), all women, were included. They shared refractory CLE forms, with a median of six previous systemic treatments (range, 1–7). All participants met the 2019 EULAR/ACR classification criteria for SLE. The most common mucocutaneous involvement was subacute CLE (SCLE) (n=9), followed by lupus perniosis (n=3), oral ulcers (n=2), and non-scarring alopecia (n=3). After 16 weeks into treatment, all patients achieved a partial response, 8 of them (88.89%) actually achieved complete responses (Fig. 1). The mean RCLASI-A reduction at 16 weeks was 26.44 points. The median time to achieve a partial response was 4 weeks (range, 4–2). The median follow-up time was 20 weeks (range, 16–48). The mean RCLASI-A reduction at the last visit was 26.78 points. Adverse events were documented in 3 patients (33.33%): upper respiratory tract infection (n=1), asthenia (n=1), and low-grade fever with asymptomatic urticarial reaction (n=1) all of mild intensity.
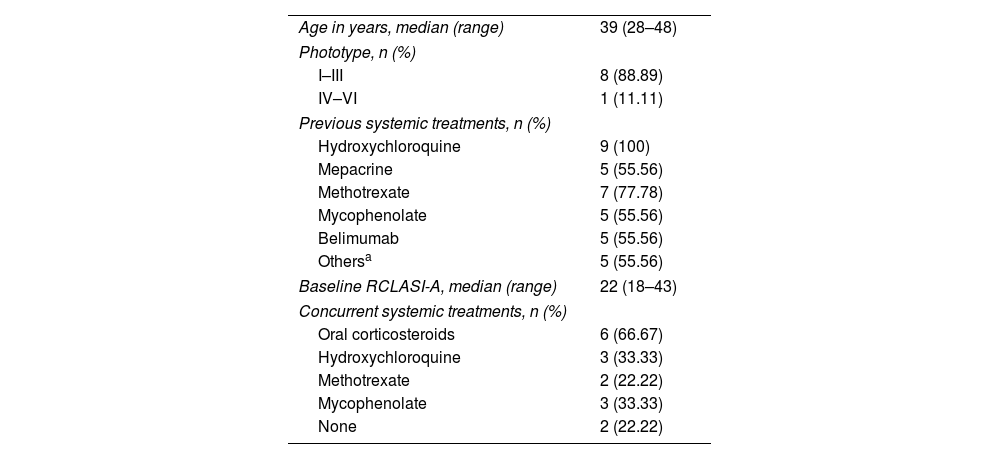
Baseline characteristics of the patients (n=9).
| Age in years, median (range) | 39 (28–48) |
| Phototype, n (%) | |
| I–III | 8 (88.89) |
| IV–VI | 1 (11.11) |
| Previous systemic treatments, n (%) | |
| Hydroxychloroquine | 9 (100) |
| Mepacrine | 5 (55.56) |
| Methotrexate | 7 (77.78) |
| Mycophenolate | 5 (55.56) |
| Belimumab | 5 (55.56) |
| Othersa | 5 (55.56) |
| Baseline RCLASI-A, median (range) | 22 (18–43) |
| Concurrent systemic treatments, n (%) | |
| Oral corticosteroids | 6 (66.67) |
| Hydroxychloroquine | 3 (33.33) |
| Methotrexate | 2 (22.22) |
| Mycophenolate | 3 (33.33) |
| None | 2 (22.22) |
SLE: systemic lupus erythematosus; RCLASI-A: Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index activity score.
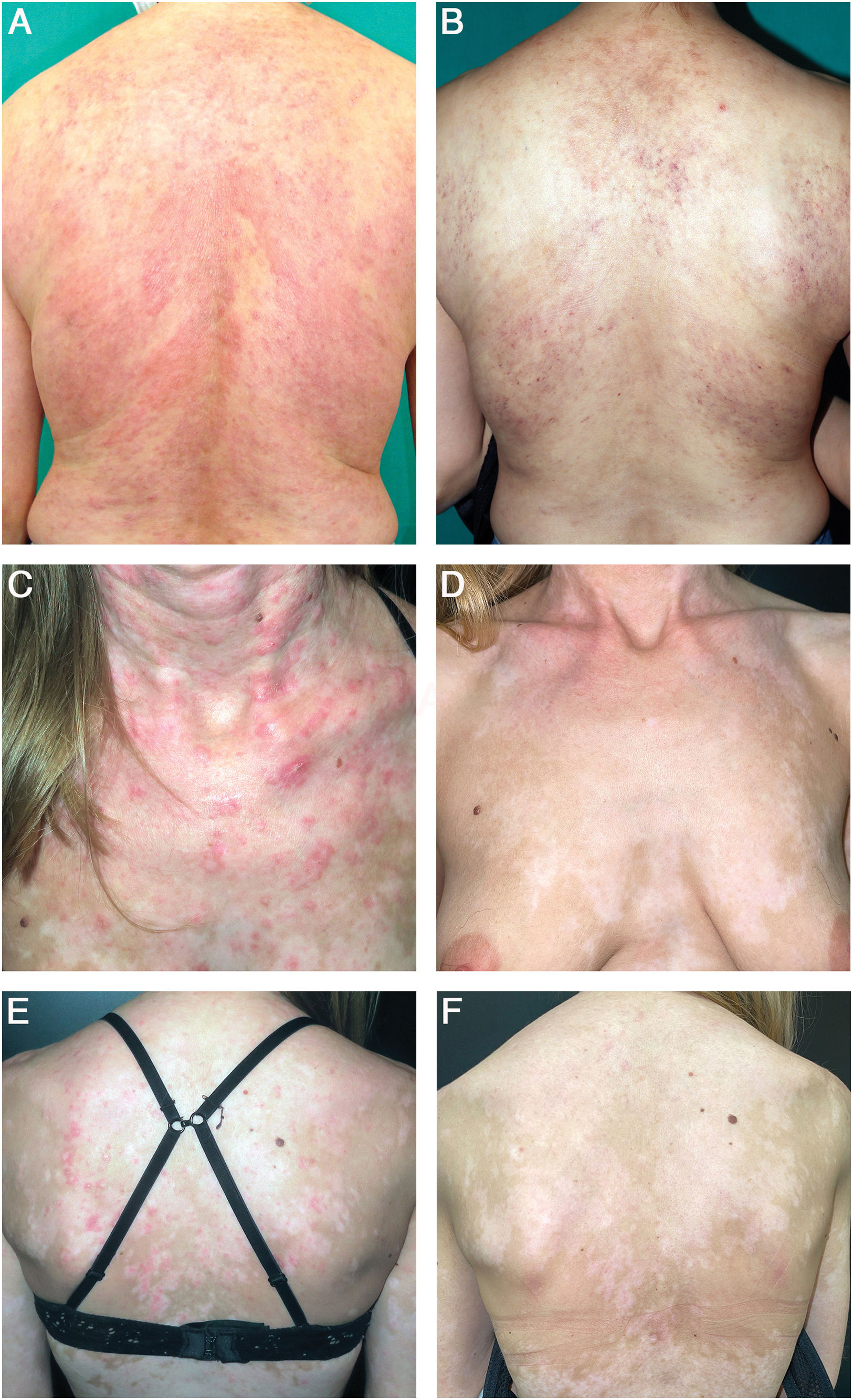
(A) Patient with SCLE lesions on the back. (B) After 16 weeks into anifrolumab treatment, the patient showed no active lesions, only residual poikiloderma. (C and D) Patient with SCLE lesions on her neck, chest, and back. (E and F) After 16 weeks into anifrolumab treatment, the patient maintained a complete response with post-inflammatory hypopigmentation.
The 2023 update of the European League Against Rheumatism (EULAR) recommendations for the management of SLE positions anifrolumab as a second-line therapy for active cutaneous disease, at the same level as methotrexate, mycophenolate, and belimumab, although with a higher level of evidence.1 A combined data analysis from the TULIP-1 and TULIP-2 trials showed anifrolumab superiority over placebo in the percentage of patients achieving a ≥50% reduction in CLASI-A at 12 weeks (46% vs 24.9%; p=0.001).2 However, the cutaneous response was a key secondary endpoint, and no information was provided regarding mucocutaneous involvement characteristics. In the past two years, multiple cases and small case series have been published highlighting anifrolumab effectiveness across a broad spectrum of CLE manifestations.
Most available evidence on the use of anifrolumab in CLE is concentrated on SCLE and discoid lupus erythematosus, in which anifrolumab has shown rapid response induction, generally observable within the first two treatment cycles.3–5 In our study, all patients achieved a partial response, and except for one, all achieved a complete response. Additionally, the rapidity of anifrolumab is highlighted, as a median of only one infusion was needed to achieve a partial response. Three patients in our series presented SCLE lesions associated with lupus perniosis lesions, a common finding due to the association of both entities with anti-Ro antibodies, which responded satisfactorily to anifrolumab. Several studies have emphasized anifrolumab utility in treating mucosal lesions, including discoid lupus erythematosus of the mucosa6 and ulcers.7,8 In our study, the 2 patients with oral ulcers showed a rapid response to anifrolumab treatment. We did not find any articles on the management of non-scarring alopecia with anifrolumab, although our experience has been positive.
The most common anifrolumab-related adverse effects in clinical trials were respiratory infections (48%), herpes zoster (6.1%), and infusion-related reactions (9.4%).9 In our study, anifrolumab was a safe and well-tolerated drug. Adverse events were observed in 3 patients (33.33%), all mild of intensity and not requiring treatment discontinuation, with no cases of herpes zoster ever being reported.
In conclusion, our study findings support anifrolumab as a rapid and effective therapeutic option for different CLE signs, with a favorable and well-tolerated safety profile. We consider anifrolumab should be regarded as a second- or third-line therapy for CLE.
FundingNone declared.
Originality confirmationThe authors confirm that the work presented in this manuscript is original and has not been published elsewhere, either in whole or in part. The content of this manuscript has not been submitted for publication to any other journal.
Patient informed consentWritten informed consent was obtained from the patients included in this study for the publication of their clinical details, images, and any identifiable information. All consent forms are in the possession of the corresponding author and are available for review by the editorial team.
Conflicts of interestNone declared.
The authors wish to thank Dr. Carlos Abril Pérez, Dr. Miguel Ángel Navarro Mira, and Dr. Iris González Villanueva for their invaluable dedication and collaboration in the preparation of this article.