Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare disease characterized by single or multiple angiomatous lesions typically located on the scalp and the face. We present a retrospective analysis of 9 cases of ALHE. The lesions appeared largely as multiple grouped papules or, in some cases, subcutaneous nodules, located mainly on the scalp, particularly around the ear. We also observed lesions in atypical locations, such as areas of the head other than the scalp, and the shoulder, neck, and forearm. At these sites the lesions had an atypical clinical appearance that made diagnosis difficult; this should be borne in mind in patients with single, well-delimited lesions with a vascular appearance and superficial ulceration or crusting. Surgery was the most common treatment in our series, and even though ALHE is considered a benign condition, recurrence was common.
La hiperplasia angiolinfoide con eosinofilia (HALE) es una enfermedad infrecuente, caracterizada por lesiones angiomatosas solitarias o múltiples que suelen localizarse en el cuero cabelludo y la cara. Presentamos un análisis retrospectivo de 9 casos de HALE. Clínicamente se manifestaban en forma de pápulas o, en algunos casos, de nódulos subcutáneos, agrupados formando lesiones múltiples localizadas en su gran mayoría en el cuero cabelludo, principalmente alrededor de la oreja. También observamos lesiones en localizaciones atípicas, como en otras áreas de la cabeza, el hombro, el cuello y el antebrazo. En estas localizaciones las lesiones adquirían una forma clínica peculiar que dificultaba su diagnóstico y que hay que tener en cuenta ante lesiones únicas, bien delimitadas, de aspecto vascular con ulceración o costra en la superficie. El tratamiento más utilizado fue la cirugía, y aunque se trate de una entidad que se engloba dentro de los procesos benignos, las recidivas fueron frecuentes.
Angiolymphoid hyperplasia with eosinophilia (ALHE) is an uncommon, benign, idiopathic disorder characterized by erythematous, violaceous, or brown papules, plaques, or nodules that arise in the skin or subcutaneous tissues. The lesions can be single or multiple and sometimes occur in groups. They may be pulsatile and they can give rise to pruritus or pain. Approximately 85% are situated on the head and neck, most commonly in the periauricular region. Although the pathogenesis of ALHE is unknown, it is considered to be a reactive disorder induced by various stimuli, including vascular malformations, trauma, and pregnancy.1 Histology reveals a clearly defined area of proliferating blood vessels with large epithelioid endothelial cells with abundant eosinophilic cytoplasm, accompanied by a lymphocytic and eosinophilic inflammatory infiltrate of variable intensity.
More recent publications on ALHE focus particularly on atypical sites such as the tongue,2 penis,3 eyelids, or orbit, and other articles describe treatments that have been successful in isolated cases, such as pulsed dye laser, intralesional bleomycin, imiquimod, and interferon alfa-2b.4 In addition, a number of recently published articles have reported evidence of clonality in some cases of ALHE, leading to a discussion of ALHE as a possible low-grade lymphoproliferative disorder rather than a reactive condition, as has been suggested up to now.5,6
The objective of the present study was to review the clinical characteristics of ALHE, focusing particularly on the atypical sites, the histology, the treatment, and the clinical course; we also compared our findings with those reported in the literature.
MethodsWe performed a retrospective study of 9 cases of ALHE diagnosed in our hospital between 1989 and 2011. The following variables were recorded: age, sex, associated diseases, site of the lesions, clinical manifestations, treatment, and clinical course. We also reviewed the histopathological findings (depth of the proliferation, intensity and composition of the infiltrate, and presence or absence of lymphoid aggregates) of all the available skin biopsies; a study of clonality had only been performed in one case.
Case DescriptionsThe clinical characteristics of the 9 patients are summarized in Table 1. There were 4 men and 5 women aged between 15 and 61 years (mean age, 39.2 years). Of note in the past medical history was that patient 3 presented human immunodeficiency virus (HIV) infection since 1997 (with a viral load at the time of diagnosis of the ALHE of <40 copies/mL and a CD4 count of 792 cells/μL) and uncomplicated hepatitis C virus (HCV) infection. This patient had also undergone surgery, radiation therapy, and chemotherapy for a cervical tumor in the year 2000, with no evidence of recurrence at the time of diagnosis of ALHE.
Clinical Data of the 9 Patients With Angiolymphoid Hyperplasia With Eosinophilia.
| Case | Age, y/Sex | Number of Lesions | Size (cm) | Symptoms | Clinical Characteristics | Lesion Site | Time Since Onset, y | Treatment | Clinical Course |
| 1 | 15/M | 5 | 0.5-1.5 | Asymptomatic, slight increase in size with fever | Brown papules and plaques | Temporal region | 1 | Intralesional corticosteroids | Resolution/spontaneous remission |
| 2 | 38/F | 13 | 0.2-3 | Pruritus and pain | Infiltrated, erythematous plaques and papules with an uneven surface | Left parietal region | 15 | Intralesional corticosteroids, RT, and surgical excision | Recurrence |
| 3 | 47/F | 2 | 4×3 | Pruritus | Papules with a tendency to coalesce into plaques | Right retroauricular region | 8 | Topical and intralesional corticosteroids | Recurrence |
| 4 | 61/M | 4 | 0.5-1.5 | Asymptomatic | Erythematous papules and nodules | Periorbital regionLeft forearmLeft shoulderRight neck | 1 | Surgical excision | Cure |
| 5 | 29/F | >2 | 0.5-1.5 | Pruritus and pain | Subcutaneous nodules with alopecia | Occipitoparietal region | 6 | Intralesional corticosteroids, surgical excision, RT | Recurrence/resolution with RT |
| 6 | 27/M | 5 | 0.5-0.7 | Pain | Violaceous papules and nodules | Occipital region | 4 | Intralesional corticosteroids, surgical excision | Recurrence |
| 7 | 53/F | 1 | NA | NA | Papule | Forehead | 1 | Surgical excision | Cure |
| 8 | 27/F | 1 | NA | NA | Papule | Glabellar region | 1 | Surgical excision | Recurrence |
| 9 | 56/M | 1 | NA | NA | Papule | Right cheek | 1 | Surgical excision | Cure |
Abbreviations: F, female; M, male; NA: not available; RT: radiation therapy.
The most common symptoms associated with the lesions were pain (in 3 cases) and pruritus (also in 3 cases).
Multiple lesions (up to 13), presenting as erythematous to brownish papules or nodules, were observed in two thirds of cases.
All patients presented lesions on the head, and in 4 cases the lesions were only observed in the periauricular region. In addition, patient 4 also presented lesions at other, less typical sites, including the shoulder, neck, and forearm.
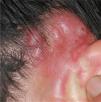
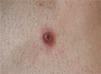
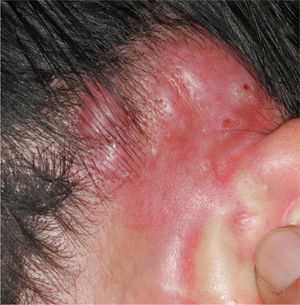
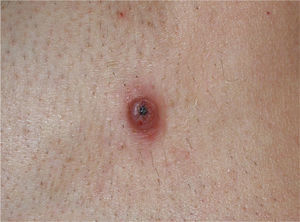
The clinical characteristics of the lesions varied depending on the site. Lesions in the temporoparietal region showed a noticeable tendency to present in groups and to coalesce into large plaques with an irregular surface (Fig. 1). In contrast, lesions at other sites were more likely to present as solitary papules with very clearly defined margins and with a superficial crust or ulcer (Fig. 2).
The time since onset of the lesions was between 1 and 15 years, with a mean of 4.2 years. Spontaneous remission with no treatment occurred in case 1; this has been reported previously in the literature.7
The first therapeutic approach in most of our cases (7 of 9) was surgical resection; clinical recurrence developed in 4 cases. Other therapeutic strategies, such as intralesional or topical corticosteroids and radiation therapy, were used in multiple or large lesions. Intralesional corticosteroids were administered in 4 patients and were effective in 2 of them. Radiation therapy was employed mainly in patients with longer-standing disease; the response was variable, with a partial response in case 2 and a complete response in case 5. Patient 3 was treated with topical corticosteroids, which improved the pruritus but did not reduce the size of the lesions.
In general, lesions outside the periauricular region had more clearly defined borders and showed no tendency to coalesce; these lesions responded better to treatment, which was surgical in most cases.
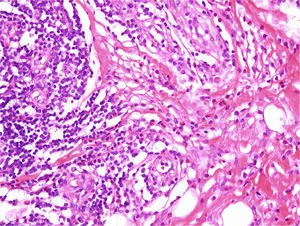
The histopathological findings were very similar in all cases and are summarized in Table 2. The tumors were predominantly intradermal, although subcutaneous forms were observed in 3 cases. They presented as well-defined, slightly lobular proliferations of capillary-sized vessels around several central vessels. In all cases, the blood vessels were lined by large endothelial cells that protruded into the lumen; these cells had abundant eosinophilic cytoplasm that often contained cytoplasmic vacuoles. Although the endothelial cells were prominent, none showed evidence of pleomorphism or of mitotic activity. An inflammatory infiltrate around the vessels was formed mainly of lymphocytes and eosinophils with isolated plasma cells and histiocytes (Fig. 3). Lymphoid nodules with no clear germinal center were detected in 3 cases in the deepest part of the dermis and in the subcutaneous tissue. The stroma was mixed fibrous and myxoid. In 2 cases we observed a few intravascular thrombi in different stages of organization. Clonality studies were only performed in case 4 and showed no evidence of a monoclonal population.
Histopathological Findings.
| Case | Intradermal ALHE | Subcutaneous ALHE | Vascular proliferation | Inflammatory infiltrate | Lymphoid aggregates |
| 1 | Yes | No | ++ | E: ++L: ++H: +P: +M: − | No |
| 2 | No | Yes | +++ | E: +++L: +++H: −P: +M: − | Yes |
| 3 | No | Yes | ++ | E: +L: ++H: −P: −M: − | No |
| 4 | Yes | No | +++ | E: ++L: ++H: −P: +M: − | Yes |
| 5 | Yes | No | +++ | E: +++L: +++H: −P: +M: − | No |
| 6 | Yes | No | +++ | E: +L: ++H: −P: +M: − | No |
| 7 | Yes | No | ++ | E: +L: ++H: −P: +M: − | No |
| 8 | No | Yes | +++ | E: +++L: ++H: −P: +++M: − | Yes |
| 9 | Yes | No | +++ | E: ++L: ++H: −P: +M: − | No |
Abbreviations: ALHE, angiolymphoid hyperplasia with eosinophilia; E, eosinophils; H, histiocytes; L, lymphocytes; M, mast cells; P, plasma cells; +, mild; ++, moderate; +++, intense.
ALHE is an uncommon idiopathic disease that usually presents as multiple tumors. The main differential diagnosis is with Kimura disease,8 which is more common in Asian patients and presents as deep nodules or subcutaneous lesions that typically affect the major salivary glands and regional lymph nodes; it is associated with elevation of the peripheral blood eosinophil count and IgE levels. Other diseases that should also be considered in the differential diagnosis include Kaposi sarcoma, angiosarcoma, epithelioid hemangioendothelioma, cutaneous epithelioid angiomatous nodule,9,10 and skin metastases.
Published reports show a slight female predominance,2,11 as was observed in our series, and the disease most commonly affects young and middle-aged individuals.2 It has been suggested that pregnancy, trauma, and vascular malformations may act as triggering or aggravating factors of ALHE,12 but we did not detect any of these factors in our patients. One of our patients had HIV and HCV infection; the association of ALHE with these infections, though extremely rare, has been reported in 2 cases published in the literature.13
We consider it important to note that lesions outside the temporoparietal region were clinically distinct from the lesions classically described in ALHE, and presented as well-defined papules with no tendency to coalesce. They occasionally showed superficial ulceration. ALHE should therefore be taken into account in the differential diagnosis of tumors with a vascular appearance outside the periauricular region.
The histology findings in our series were similar to those described in the literature. Subcutaneous forms were observed in 3 cases. Immunohistochemistry showed that the majority of the lymphocytes were T cells, but there were also occasional B cells forming the lymphoid aggregates.7
Although the natural history of ALHE is as a benign, slow-growing lesion, recurrence is common after surgery and occurred in 4 of our patients. Nonconfluent lesions less typical of ALHE responded better to surgery and were less likely to recur; this may have been because the lesions were more clearly defined.
Spontaneous remission of ALHE has been reported previously14 and, though rare, it was observed in 2 lesions in one of our patients.
There are recent reports of complete remission after treatment with intralesional corticosteroids,15 but this treatment was only effective in half of the patients in whom it was used in our series, perhaps because treatment was not continued as long as in the published reports, in which monthly injections were administered for 6 months. A few cases have been treated successfully with radiation therapy, particularly in complex areas such as the hand.16 Laser therapy has also been used to treat ALHE lesions17; we did not use this technique as it is not available in our hospital.
No associated malignant lesions were detected during follow-up (1-15 years).
The pathogenesis of ALHE has not been fully defined, and there is an on-going discussion about whether it is a reactive disorder or a true vascular neoplastic disease. Some authors have detected human herpesvirus 8 in ALHE lesions,18 but results have not been consistent.19,20 Miteva et al.21 suggested a possible lymphatic component in ALHE given the positivity for D2-40 in the histology of one case, though this finding has not been corroborated in other studies.
Recently, clonal T-cell populations have been detected in a number of cases of ALHE,5,6 and the authors of those studies therefore suggested that some subtypes of ALHE could have a T-cell origin and could be a benign or low-grade malignant lymphoproliferative disorder.17 We were only able to analyze this aspect in one of our patients and we found no evidence of clonality.
In summary, although the clinical and histological data from the patients and lesions in our case series were very similar to those published in the literature, we would like to draw particular attention to the existence of atypical sites in which the presentation differs from typical ALHE, complicating the diagnosis. This disease must be considered in patients with isolated, well-defined lesions with a vascular appearance and superficial ulceration or crusting.
Although this disease is considered to be a benign reactive disorder, its persistence over time, its poor response to available treatments, the frequency of recurrence, and the finding of clonality in some cases have given rise to numerous uncertainties concerning its etiology and pathogenesis. Clarification of whether AHLE is a reactive or neoplastic disease is one of the enigmas to be resolved, and the low prevalence of the condition means that multicenter studies will be required to further our understanding not only of its etiology and pathogenesis but also of its treatment.
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to Privacy and Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr. Fernández-Figueras for generously allowing us to use the histology images.
Please cite this article as: Guinovart R, Bassas-Vila J, Morell L, Ferrándiz C. Hiperplasia Angiolinfoide con Eosinofilia. Estudio Clínico Patológico de 9 Casos. 2014;105:e1–e6.