Invasive fungal infections are an increasingly important problem in immunocompromised hosts, with a high mortality rate.1
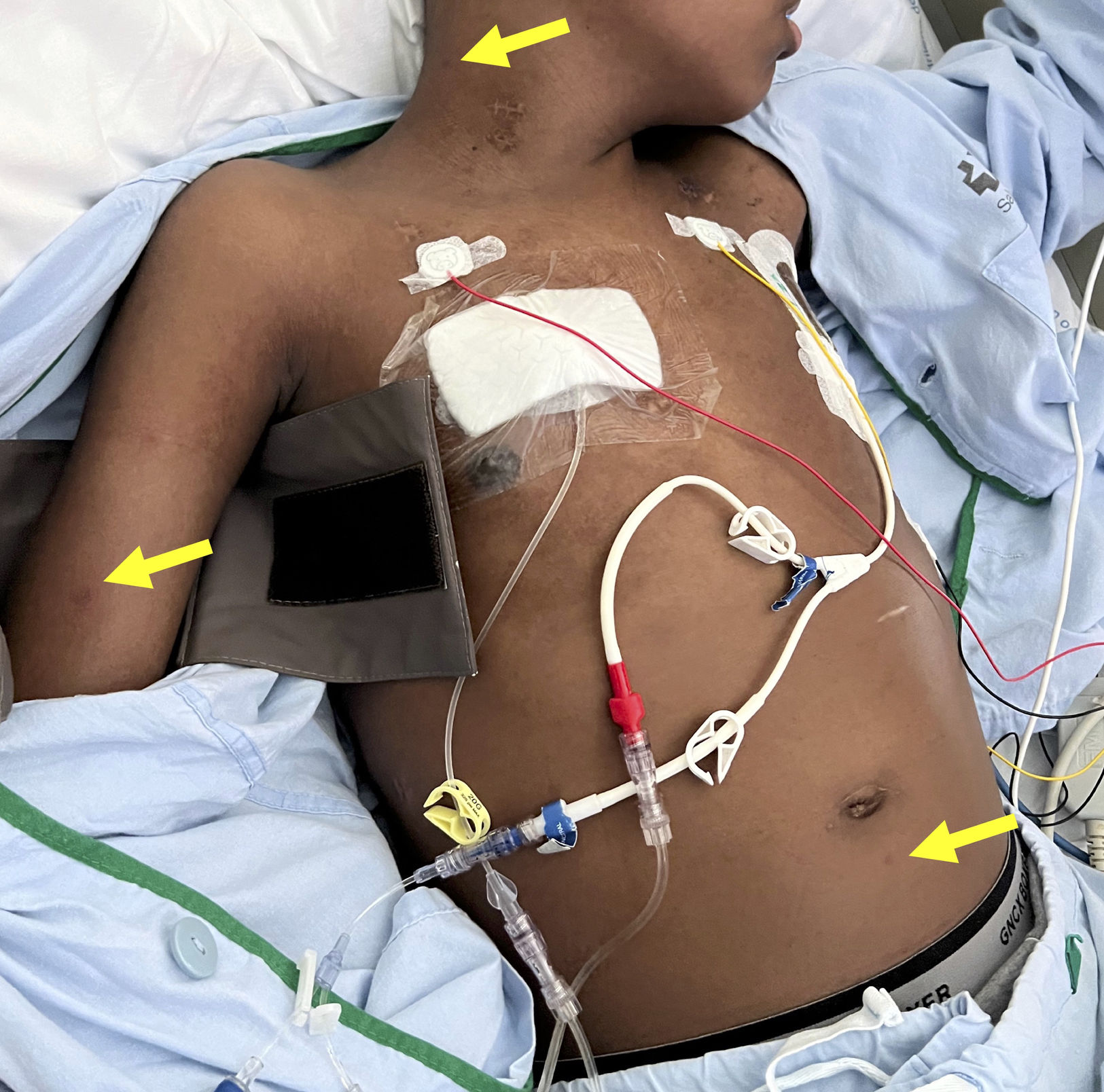
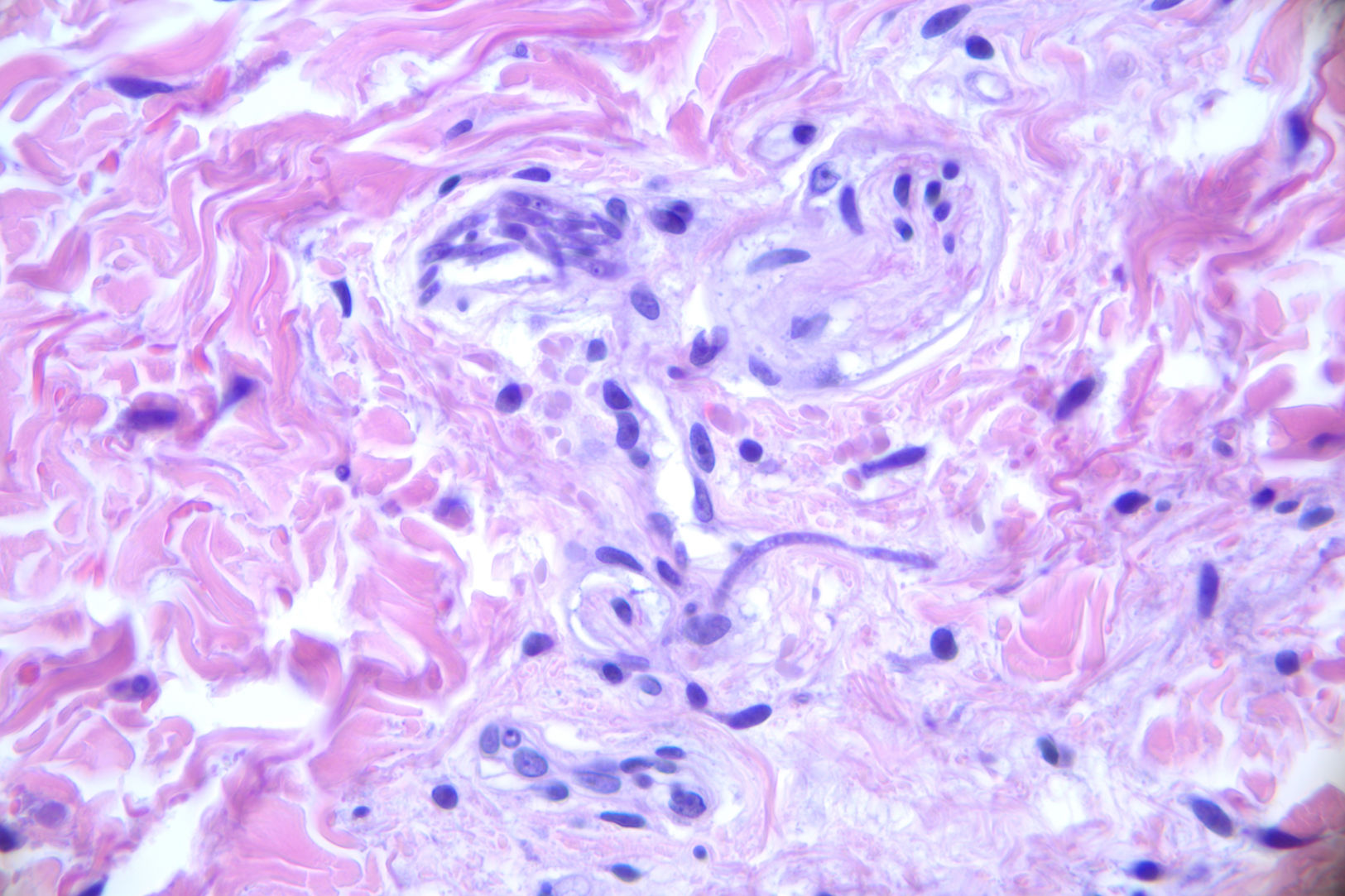
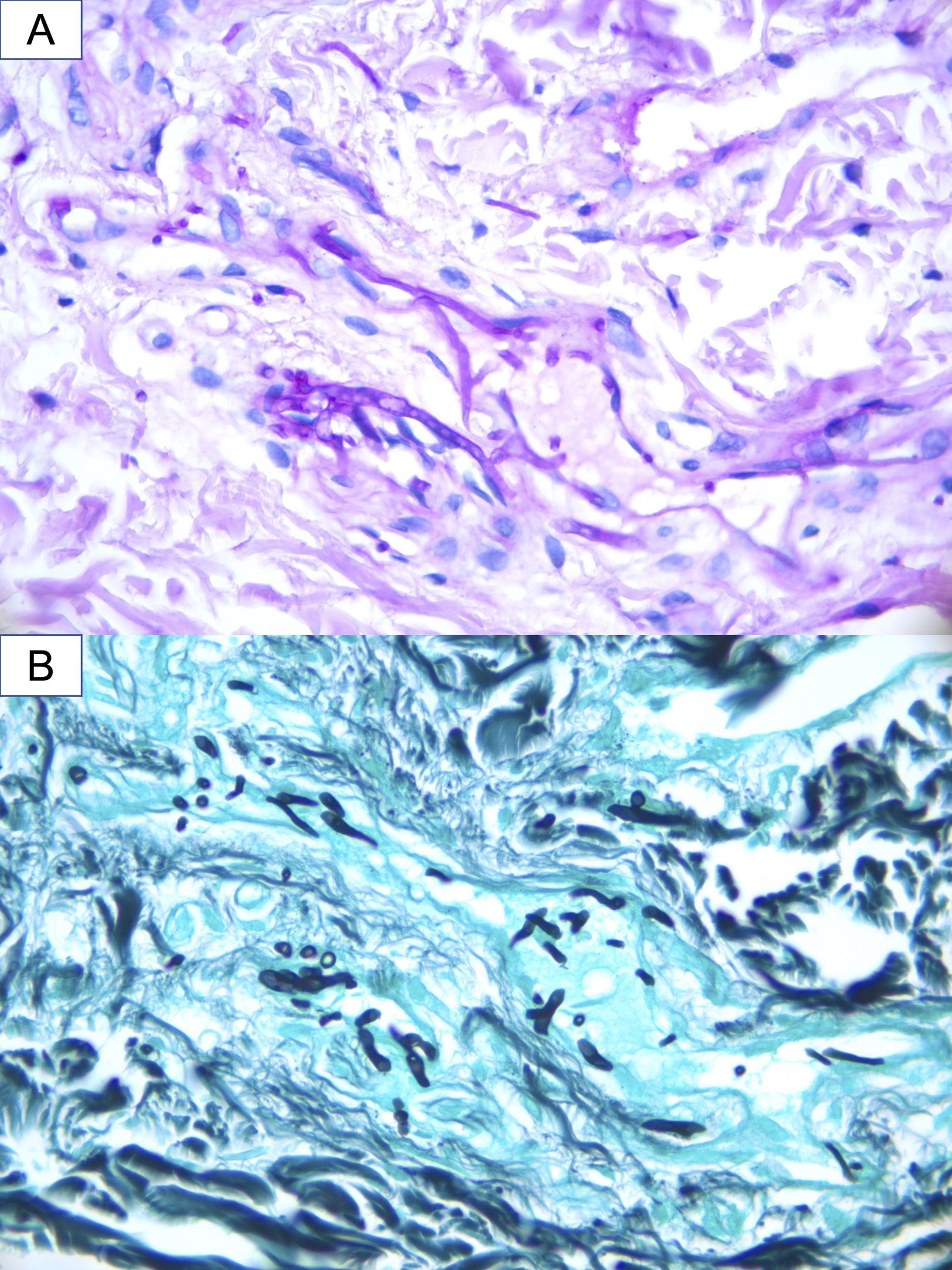
A 16-year-old hospitalized adolescent diagnosed with sickle cell anemia with a 1-day history of 4 purpuric nodules, 5mm in diameter, located on the neck, right upper limb, and abdomen (Fig. 1). The patient had undergone two consecutive hematopoietic progenitor transplants, the last one 9 days prior, and had neutrophil counts of 0 per microliter, so he was on prophylactic therapy with liposomal amphotericin B at 3mg/kg/day. He remained afebrile. The patient had experienced consolidative pneumonia a few days before, with a bronchoalveolar lavage PCR negative for fungi, although the fungal marker galactomannan had increased from 0.17 up to 5.71. Due to suspicion of invasive fungal infection, biopsies were performed for calcofluor staining, histological examination and fungal culture. Blood cultures were also taken. The calcofluor staining revealed fungal structures, so the dose of amphotericin B was increased to 5mg/kg/day, and isavuconazole was added. The hematoxylin–eosin staining showed, in the middle dermis, a group of hyaline and septate hyphae located intravascularly and immediately adjacent to the vessel (Figs. 2 and 3). The fungal culture grew Fusarium verticillioides sensitive to amphotericin B, posaconazole, and voriconazole. Blood cultures all tested negative. Isavuconazole was switched for voriconazole, and filgrastim was administered, resulting in a partial recovery of neutropenia, an excellent clinical response, resolution of the condition, and a reduction in galactomannan levels down to 0.18.
Invasive fungal infections are a serious problem in immunocompromised patients, particularly in those with severe and prolonged neutropenia. Antifungal resistance associated with prophylactic use has exacerbated this problem.2
TOpportunistic fungi responsible for these infections include yeasts (such as Candida spp.) and filamentous fungi. The latter include Aspergillus spp., agents of mucormycosis (such as Rhizopus, Mucor, or Rhizomucor), hyalohyphomycosis (such as Fusarium), and pheohyphomycosis (such as Alternaria). These fungi are ubiquitous in the environment and rarely cause infections in immunocompetent individuals. However, in neutropenic patients, they can invade blood vessels, causing ischemia, infarction, and tissue necrosis, including skin necrosis.3
In disseminated Fusarium infections, skin lesions are present in more than 70% of patients.4 These lesions are typically multiple and widespread. They include red or gray macules or papules, some with central necrosis or eschar resembling ecthyma, purpuric papules, pustules, or subcutaneous nodules. This clinical presentation results from thrombosis of dermal vessels by Fusarium hyphae, subsequent extravasation of erythrocytes, and further focal dermal necrosis and epidermal ulceration.1 Patients often present with myalgias and persistent fever unresponsive to empirical antibiotic and antifungal treatment.5
Once the infection has been suspected, the diagnostic process should begin immediately, including biopsy with an immediate fungal stain (such as calcofluor or KOH), histological examination, fungal culture, blood cultures, and radiological tests to assess pulmonary and sinus involvement.1 Blood cultures are only positive in 40% of patients with disseminated fusariosis,6 possibly due to the use of prophylactic antifungals or sample collection conditions. Definitive diagnosis requires histology demonstrating fungal structures inside the vessels, which explains the need for urgent microscopic examination, either with rapid stains or frozen histological sections. Nevertheless, culture is key to identifying the type of fungus (as Aspergillus and Fusarium appear identical under the microscope) and determining its sensitivity.
Regarding the treatment of invasive fusariosis, there are no studies comparing the efficacy of monotherapy vs combined therapy. However, poor responses to monotherapy have favored the use of combination treatments.7Fusarium is intrinsically resistant to echinocandins and flucytosine, and in our setting, it shows greater sensitivity to amphotericin B and voriconazole than to isavuconazole.7,8 The combination proposed by the guidelines is amphotericin B along with voriconazole.9 However, what truly impacts prognosis is the recovery of neutropenia, so leukocyte transfusions and growth factors should also be considered.1 The mortality rate of patients with disseminated infection is 80%.1
For prevention, it is necessary to control potential entry points for the fungus. The primary route is airborne, followed by injured skin and mucous membranes. Environmental sources of Fusarium, such as tap water, should be avoided, and surface cleaning should be thorough, using detergent followed by disinfectant.10 Injured skin is responsible for 33% of cases, typically paronychia or traumatic ulcers. Fusarium-related onychomycosis is estimated to be underdiagnosed and represents 6% of onychomycosis cases in immunocompetent patients.1 Careful examination of the nails and skin lesions is important, with culture collection and appropriate antifungal therapy. Antifungal prophylaxis in neutropenic patients is important but often insufficient. In one series, 50% of patients with disseminated fusariosis were on prophylactic or empirical antifungal treatment, at least, 1 week before diagnosis,1 as occurred in our case.
In conclusion, in a neutropenic patient with skin lesions consistent with angioinvasive mycosis, it is important to perform rapid diagnostic evaluation and initiate treatment immediately, which should include high-dose antifungal therapy and measures to recover neutropenia.