Basal cell carcinoma (BCC) is the most common type of skin cancer. A minority of patients develop locally advanced tumors or, less frequently, metastases.1 Hedgehog pathway inhibitors (vismodegib and sonidegib) are the treatment of choice for advanced BCC when radiotherapy or surgery is contraindicated. However, up to 80% of patients experience adverse effects, which limits their use.2 The increase in life expectancy has led to a larger elderly population with specific physiological and medical characteristics that affect the diagnosis and treatment of skin cancer.3 Pharmacokinetic and pharmacodynamic differences in older adults could influence and safety and efficacy profile of treatment. To date, no studies have ever compared whether the adverse effects of sonidegib differ between younger and older patients in real-world clinical settings. Real-life safety data on sonidegib may help personalize treatment according to age.
ObjectiveTo describe and compare the adverse effects of sonidegib in patients aged≥85 years and <85 years, determining whether there are differences in the frequency, type, and severity of side effects.
MethodsWe conducted a multicenter, retrospective, observational, descriptive study with patients with advanced BCC on a 3-month regimen of sonidegib for more than. The study period spanned from February 2019 to February 2024. Clinical, epidemiological, and safety data were collected, and a descriptive analysis comparing both groups was performed.
ResultsA total of 119 patients were included: 65 aged≥85 years (51% men) and 54 aged<85 years (54% men). The mean age was 90.2 and 65.5 years, respectively.
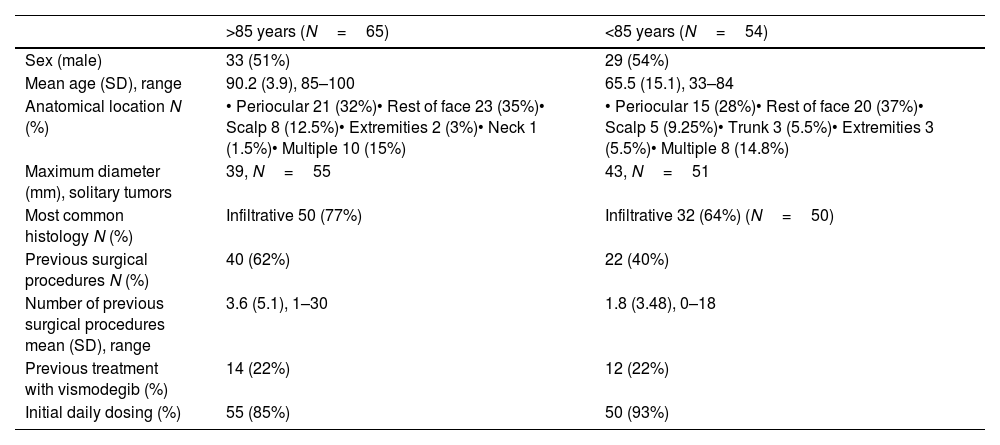
The most frequent tumor location was the periocular region in both groups (32% of cases in those ≥85 years and 28% in those <85 years). The mean tumor diameter was 39mm in older patients and 43mm in younger patients. The most common histological subtype was infiltrative (77% in ≥85 years vs 64% in <85 years). Regarding prior treatments, 62% of patients aged≥85 years and 40% of those <85 years had undergone surgery. In the 2 groups, 22% had previously received vismodegib. The initial sonidegib dosage was daily in 85% of the ≥85-year group and 93% of the <85-year group (Table 1).
Clinical, histological, and epidemiological characteristics of patients.
| >85 years (N=65) | <85 years (N=54) | |
|---|---|---|
| Sex (male) | 33 (51%) | 29 (54%) |
| Mean age (SD), range | 90.2 (3.9), 85–100 | 65.5 (15.1), 33–84 |
| Anatomical location N (%) | • Periocular 21 (32%)• Rest of face 23 (35%)• Scalp 8 (12.5%)• Extremities 2 (3%)• Neck 1 (1.5%)• Multiple 10 (15%) | • Periocular 15 (28%)• Rest of face 20 (37%)• Scalp 5 (9.25%)• Trunk 3 (5.5%)• Extremities 3 (5.5%)• Multiple 8 (14.8%) |
| Maximum diameter (mm), solitary tumors | 39, N=55 | 43, N=51 |
| Most common histology N (%) | Infiltrative 50 (77%) | Infiltrative 32 (64%) (N=50) |
| Previous surgical procedures N (%) | 40 (62%) | 22 (40%) |
| Number of previous surgical procedures mean (SD), range | 3.6 (5.1), 1–30 | 1.8 (3.48), 0–18 |
| Previous treatment with vismodegib (%) | 14 (22%) | 12 (22%) |
| Initial daily dosing (%) | 55 (85%) | 50 (93%) |
SD: standard deviation.
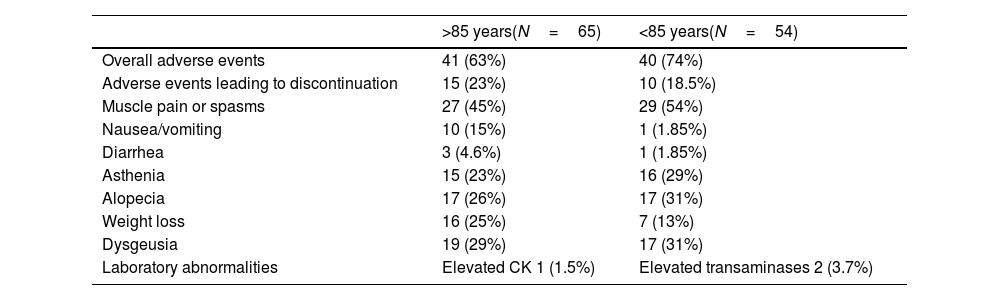
Overall, 63% of patients aged≥85 years and 74% of those <85 years experienced at least 1 adverse event, leading to treatment discontinuation in 23% and 18.5% of cases, respectively. Muscle spasms or pain occurred in 45% of older patients and 54% of younger ones. Nausea or vomiting was reported in 15% of older patients and in 1.85% of younger ones, while diarrhea occurred in 4.6% and 1.85%, respectively. Asthenia affected 23% of patients aged≥85 years and 29% of those <85 years. Alopecia and dysgeusia were slightly more frequent among younger patients (26% vs 31% and 29% vs 31%, respectively). Weight loss occurred in 25% of older adults compared with 13% of younger patients. Laboratory abnormalities were rare: one patient aged≥85 years showed elevated CK levels (1.5%), and 2 patients aged<85 years presented elevated transaminases (3.7%) (Table 2). Statistical analysis revealed no trends or statistically significant differences.
Frequency of adverse effects in both groups.
| >85 years(N=65) | <85 years(N=54) | |
|---|---|---|
| Overall adverse events | 41 (63%) | 40 (74%) |
| Adverse events leading to discontinuation | 15 (23%) | 10 (18.5%) |
| Muscle pain or spasms | 27 (45%) | 29 (54%) |
| Nausea/vomiting | 10 (15%) | 1 (1.85%) |
| Diarrhea | 3 (4.6%) | 1 (1.85%) |
| Asthenia | 15 (23%) | 16 (29%) |
| Alopecia | 17 (26%) | 17 (31%) |
| Weight loss | 16 (25%) | 7 (13%) |
| Dysgeusia | 19 (29%) | 17 (31%) |
| Laboratory abnormalities | Elevated CK 1 (1.5%) | Elevated transaminases 2 (3.7%) |
CK: creatine kinase.
In our study, both groups exhibited fewer adverse effects compared with phase II trials and previous real-world studies.4–6 Although no major differences were observed across different age groups, advanced age may be associated with better tolerance to muscular adverse effects due to lower physical activity levels. However, weight loss was more frequent in older adults, possibly related to comorbidities or frailty. The proportion of patients who discontinued treatment due to adverse effects was slightly higher in the ≥85-year group, which may reflect greater tolerance or willingness to continue therapy among younger patients.
The latency of the various adverse effects was similar to that reported in former studies,6 although, except for muscle spasms, all adverse events showed longer latency in older adults (Table 3).
Latency of different adverse effects (weeks).
| Latency (weeks) | >85 years(N=65) | <85 years(N=54) |
|---|---|---|
| Muscle spasmsMean (SD), Range | 9.2 (7.0), 1–22 | 10.15 (6.2), 2.1–25.7 |
| DiarrheaMean (SD), Median [IQR] | 3.3 (2.0), [1–5] | 2.14a |
| FatigueMean (SD), Median [IQR] | 12.5 (9.5), [1–30] | 8.1 (7), 5.85 [3.8–9.5] |
| AlopeciaMean (SD), Median [IQR] | 24.8 (15.4), [4–50] | 14.7 (9.3), 12.85 [8.5–17.1] |
| Weight lossMean (SD), Median [IQR] | 17.8 (8.1), [4–30] | 16.1 (5.9), 18.5 [10.5–21.4] |
| DysgeusiaMean (SD), Median [IQR] Range | 11.2 (7.9), 8.0 [4.0–17.0] 1–3.71 | 10.1 (9.9), 8.71 [4.28–11.42] 4.28–11.42 |
| Nausea or vomitingMean (SD), Median [IQR] | 12.2 (7.9), [2–28] | 25.71a |
Study limitations include its retrospective design, intercenter heterogeneity, and limited sample size.
ConclusionsIn conclusion, sonidegib seems to have a favorable tolerability and safety profile comparable between younger and older patients, with no clinically relevant differences observed in real-world practice. Advanced age should not be considered a limiting or restrictive factor for its use.
Conflict of interestThe authors declare that they have no conflict of interest.
Ignacio Torres-Navarro (Valencia), Montserrat Bonfill-Ortí (Barcelona), Verónica Ruiz-Salas (Barcelona), Emili Masferrer (Barcelona), Luisa Martos-Cabrera (Madrid), Gustavo Deza (Barcelona), Ricardo Fernández-de-Misa Cabrera (Santa Cruz de Tenerife), Carlos Feal (Pontevedra), Lucía Turrión-Merino (Madrid), Agustín Toll (Barcelona), Carlos Abril Pérez (Valencia), Rafael Botella Estrada (Valencia), Mireia Yébenes (Barcelona), Sagrario Galiano-Mejías (Madrid), David Jiménez-Gallo (Cádiz), Carla Ferrandiz-Pulido (Barcelona), Ángeles Flórez (Santiago de Compostela), Noelia Hernández-Hernández (Santa Cruz de Tenerife), Luis Ríos-Buceta (Madrid), Onofre Sanmartín (Valencia), Cristina Ciudad Blanco (Madrid), Ane Jaka (Barcelona), Miquel Just-Sarobé (Tarragona), Alberto Conde-Taboada (Madrid), Matías Mayor Arenal (Madrid), Elena Vargas-Laguna (Madrid), Inmaculada Alcaraz León (Madrid), Lorena Leal (Barcelona), Susana Medina Montalvo (Madrid), Isabel Polo (Madrid), Berta Hernández-Marín (Madrid), Ainara Soria (Madrid), and Yolanda Delgado-Jiménez (Madrid).