Daylight PDT (dPDT) is easy to use and does not require light equipment. Such therapy has been exhaustively proved to be successful in the treatment of actinic keratosis, but its use in skin photodamage remains unclear.
ObjectiveTo evaluate dPDT's efficacy in skin facial photodamage.
Patients and methodsThis was a parallel-group double-blind, randomized placebo-controlled trial. Sixty participants with symmetric facial photodamage were allocated to topical methyl aminolevulinate (MAL) and daylight vs. matching placebo and daylight. Primary outcome was global photodamage improvement/failure 1 month after the third session. Secondary outcomes included: pain evaluation; specific photodamage severity scores; sun irradiance quantification and Skindex-29 scores. Adverse events were also investigated.
ResultsPrimary analysis included all randomized patients. All patients sun-exposed for 120min in 3 sessions. The risk of failure was lower in the MAL-dPDT group than in the placebo plus daylight group (RR: 0.18; 95% CI: 0.08–0.41). Mean solar irradiance (W/m2) during the first, second and third sessions was 480.82, 430.07 and 435.84, respectively. Items 5 and 14 of Skindex-29 in the MAL-dPDT group showed statistical significant differences. Two patients in the MAL-dPDT group had serious and non-serious events not directly related to the product.
ConclusiondPDT with MAL was un-painful, effective and safe for the treatment of facial photodamage. Herpes simplex prophylaxis should be considered before sessions.
La terapia fotodinámica con luz-día (TFDd) es fácil de usar y no requiere de equipo alguno. Tal terapia ha demostrado ser útil en el tratamiento de las queratosis actínicas, pero su uso en el fotodaño no es claro.
ObjetivoEvaluar la eficacia de la TFDd en el fotodaño facial.
Pacientes y MétodosSe realizó un ensayo clínico doble-ciego controlado con placebo y con asignación aleatoria. Sesenta participantes con fotodaño facial simétrico se asignaron a recibir bien TFD con Metil-Aminolevulinato (MAL) y luz de día o placebo y luz de día. El resultado primario fue la mejoría/fracaso en el fotodaño facial global un mes después de la tercera sesión. Los resultados secundarios incluyeron: dolor; fotodaño específico, irradiancia recibida y la puntuación en el Skindex-29.
ResultadosTodos los pacientes se expusieron a la luz de día durante 120 minutos en 3 sesiones. El riesgo de fracaso fue menor en el grupo de TFD con MAL y luz de día que en el grupo placebo (RR:0,18; 95%; IC:0,08 a 0.41). La media de la irradiancia solar (W.m-2) durante la primera, segunda y tercera sesión fue de 480,82, 430,07 y 435,84, respectivamente. Los ítems 5 y 14 del Skindex-29 en el grupo de TFDd con MAL mostraron diferencias estadísticamente significativas. Dos pacientes en el mismo grupo presentaron eventos adversos serios y no serios pero estos no tuvieron relación directa con el producto evaluado.
ConclusiónLa TFDd con MAL fue es un tratamiento indoloro, eficaz y seguro para el tratamiento del fotoenvejecimiento facial. La profilaxis del Herpes simple debe ser considerada antes de cada sesión.
As worldwide population's average age has risen,1 skin care and self-perception concerns have both increased.2 Chronic sun or ultraviolet (UV) light exposure cause physical and structural changes to the skin that result in photodamage,3 which could also be a marker for the development of actinic keratosis or skin cancer.
Available treatments for photodamage have included multiple procedures such as topical and systemic retinoids, chemical peels, intense-pulsed light, lasers, and photodynamic therapy (PDT).4–7 With the exception of PDT,8 published evidence of such procedures efficacy in photodamage is lacking.9
Photodynamic therapy is based in the use of photosensitizers activated by light which localize in the diseased cells resulting in the formation of reactive oxygen species that leads tissue damage and cell death.10 The most often used topical photosensitizers for the skin are 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL) which are endogenously converted to protoporphyrin-IX (PpIX).11
Conventional PDT (cPDT) relies on the incubation of any of these photosensitizers with occlusion for several hours, with severe pain during illumination as a main disadvantage of the procedure.8,12
Daylight PDT (dPDT) is easy to use and less expensive due to the lack of need of light equipment.11,13,14 It has also been described to be better tolerated by patients, as continuous activation of porphyrins during daylight exposure can lead to less pain.15 Such therapy has been exhaustively proved to be successful in the treatment of actinic keratosis,13,15,16 but its use in skin photodamage remains unclear. Therefore, the aim of this study was to evaluate the efficacy of dPDT vs. placebo in adult patients with facial photodamage in terms of failure and improvement according to Dover's et al. scale.7 Such study contributes with trial evidence in this field, as it has been confirmed that daylight-mediated photodynamic therapy is possible throughout the year in Medellin, Colombia, according to a recent meteorological study performed in Central and South America.17
Patients and methodsPatientsPatients screened belonged to an ambulatory clinic (IPS Universitaria, Universidad de Antioquia). All adult patients, willing to participate, between 35 and 75 years-old with symmetric facial photodamage grade 2 or 3 according to Dover's scale, were included. Exclusion criteria were nursing or pregnancy; photosensitizing disorders; active infectious skin diseases or history of herpes simplex in the face; subjects with less than 6 months of any previous rejuvenation interfering treatments; history of systemic isotretinoin in the last year; history of hypersensitivity to the active product; and subjects requiring concurrent treatment that would have interfered with the aims or assessments of the study. All patients were enrolled by one dermatologist, and each eligible patient was sequentially assigned with a number starting from 1.
Design and randomizationThis was a phase IIb-trial designed to elucidate mainly dPDT's efficacy in another indication such as skin photodamage, as it has been previously proved to be effective in the treatment of Actinic Keratosis (AKs).13,15,16
This was a unicentre Phase II, 2 arms, parallel group double blind, randomized placebo-controlled trial. Sixty participants were allocated using a ratio of 1:1 to receive either topical MAL (Metvix®, Galderma Laboratories, France) plus daylight or topical matching placebo-daylight. Allocation sequence was generated by an external statistician through a simple random sampling without replacement.18
Allocation concealment was warranted by sending the generated sequence by the statistician to the pharmacist chemist who was entailed to label and supply the active intervention and matching placebo according to a “A” or “B” code's assignment list. This coded list was thereafter sent by the pharmacist chemist to the nurse in charge of the application of the topicals who did not know the generated sequence.
SettingThe study setting and data collection was held in one center in Medellin, Colombia at an ambulatory clinic (IPS Universitaria, Universidad de Antioquia).
EthicsStudy approval was obtained from the Ethics Committees/Institutional Review Boards at the participating centre. The study was designed to follow the International Conference on Harmonization of good clinical practice (GCP) guidelines, local regulations and laws, as well as to conform to Helsinki Declaration. All participants gave written informed consent before study start.
InterventionsPatients were randomized to receive 1g of topical MAL or matching placebo applied to the whole face <30min before sun exposure for 2h (3 sessions, 2–4 weeks apart) in a double-blind fashion (investigators and patients).
To enhance product/placebo skin penetration a subtle abrasion with sandpaper 400 grit to the whole face, was performed. Immediately after skin abrasion, a sunscreen (Cetaphil Dermacontrol SPF30®) was applied to the entire sun-exposed area including the treatment area in both groups during daylight-PDT, to avoid sunburn. Thereafter, 15min after sunscreen application, MAL was applied.
If ambient temperature and/or sunny sky were uncomfortable for the patients, they were allowed to stay under a gazebo. Also, patients receiving placebo were allowed to receive the active intervention after data analysis and prove of efficacy.
Safety assessmentsPatients were assessed for safety one week after each session by a Dermatologist. Patients were monitored for adverse events using INVIMA's (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) criteria and serious adverse events were reported in the first 24h after knowledge of the event. Pain after each session was assessed by a trained nurse.
Efficacy assessmentsEfficacy was evaluated after 1 month of the third (last) daylight session by another dermatologist not involved in assessing safety secondary outcomes.
Assessment of light doseAmbient temperature, daylight illuminance and irradiance were measured during all 3 sessions with LP-471 probes connected to a Delta-Ohm 9847 data-logger (Caselle di Selvazzano (PD), Italy), which performed measurements every minute starting from the time the first patient started daylight exposure, until the last patient ended exposure. This equipment was calibrated by the manufacturer.
Primary outcomesThe primary outcome was measured with the Dover's photodamage scale,7 1 month after the third daylight PDT session. According to a previous publication,8 outcome was labeled as “success if there was a decrease in global photodamage score to a severity score of 0 or if there was a >1 grade of decrease in global photodamage score from baseline. A failure or lack of improvement was considered if, after therapy, the patient had the same severity score found at baseline”.
Secondary outcomesSecondary outcomes included: pain evaluation with the visual analog scale (VAS) measured immediately after sessions 1, 2 and 3; specific photodamage severity score for fine lines, coarse lines, tactile roughness, mottled pigmentation, sallowness, and erythema measured one month after the third daylight PDT session, according to Dover's photodamage scale7; and sun irradiance quantification during daylight exposure. Another secondary objective was quality of life assessment before/after treatment measured with the validated version of the Colombian Skindex-29 Instrument. Secondary safety objectives included assessment of any adverse event at all times, and therapy tolerance measured 1 week after sessions 1, 2 and 3. No changes in trial outcomes were added after the start of the trial.
Study variablesGlobal and specific photodamage variables (fine lines, mottled pigmentation, sallowness, tactile roughness, coarse lines, and erythema) were measured by Dover's photodamage scale (Severity score: from 0 to 5).
Pain was measured by the quantitative visual analog scale (rated from 0 to 10).
Therapy tolerability (oozing, erythema, edema, desquamation, pigmentation and vesiculation) was measured 1 week after session 1 and session 2 (rated from 0 to 3).
In all variables, absolute and relative values were depicted, accordingly.
Statistical analysisA minimum of 58 patients was required to give the study at least 80% power at a two-tail 5% level of significance to detect a difference in proportions of the primary outcome of 70% with MAL-dPDT vs. 30% with placebo-dPDT. These calculations were performed using Epidat®.19
All randomized patients (intention to treat (ITT) population) were included in the primary analysis. The population considered for efficacy and safety analysis consisted in all patients who received at least one dose of the study medication.
Efficacy was assessed with the calculation of the relative risk (RR) of failure with its respective 95% confidence interval. Differences between proportions of primary outcome and qualitative secondary endpoints were assessed using the χ2 test or Fisher exact test, as required. When more than 50% of cells were found to have ceros a test for comparison of proportions, was performed. Wilcoxon signed rank test was used to compare global facial photodamage and secondary outcomes severity scores on each patient, at baseline and 1 month after session 3, and the U-Mann Whitney test was used to compare pain differences.
Post hoc logistic regression to evaluate confounding and subgroup-treatment effect interactions was performed, according to gender, skin phototype, sun irradiance and time between sessions. The effect of the interventions was also evaluated adjusting for age, sun irradiance and time between sessions.
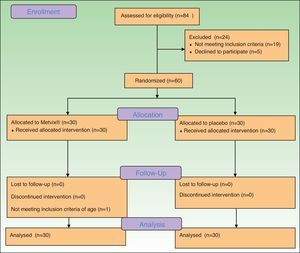
ResultsA total of 84 patients were initially screened but from these, 19 did not fulfill eligibility criteria and 5 refused to participate, obtaining 60 eligible participants. (Fig. 1) The first patient was enrolled on April 10th 2014, and the last patient completed the study on the 3rd of October 2014. The trial was registered at http://clinicaltrials.gov with the identifier: NCT02139618.
Demographic and clinical baseline characteristics of patients are depicted in Table 1.
Baseline characteristics of patients.
| Variables | Placebo+daylight (n=30) | MAL+daylight (n=30) | p value | 95% CIa |
|---|---|---|---|---|
| Age | 60 (sd: 7.6) | 60.5 (sd: 8.1) | 0.62 | −3.55 to 4.55 |
| Gender | ||||
| Males | 5 (17%) | 1 (3%) | 0.20 | −4% to 31% |
| Females | 25 (83%) | 29 (97%) | 0.58 | −31% to 4% |
| Skin phototype | ||||
| I | 1 (3%) | 0 (0%) | 0.68 | – |
| II | 18 (60%) | 15 (50%) | 0.50 | −18% to 38% |
| III | 11 (37%) | 15 (50%) | – | −41% to 14% |
| IV | 0 (0%) | 0 (0%) | – | |
| V | 0 (0%) | 0 (0%) | – | |
| VI | 0 (0%) | 0 (0%) | – | |
| Dover's global photodamage score | ||||
| 1 | 0 (0%) | 0 (0%) | – | – |
| 2 | 6 (20%) | 3 (10%) | 0.48 | −11% to 31% |
| 3 | 24 (80%) | 27 (90%) | 0.7 | −31% to 11% |
| 4 | 0 (0%) | 0 (0%) | – | – |
The ITT analysis included all 60 patients (54 females/6 males) for the primary outcome, according to allocation. As there were no exclusions or patients lost to follow-up, all 60 individuals were included in all other analyses.
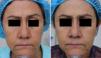
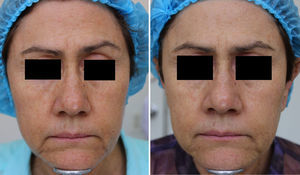
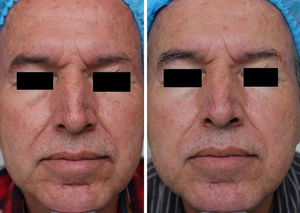
MAL-dPDT was found to have a significantly greater treatment effect than placebo-daylight, with the majority of patients of the first group having facial improvement (15 out of 30) and 10 out of 30 having facial success vs less patients of the placebo group having facial improvement (2 out of 30) and 1 out of 30 having facial success (p=0.00). Clinical effects are shown in Figs. 2 and 3.
For RR calculation, the number of whole faces that succeeded was added to the number of whole faces that improved. The risk of failure was lower in the MAL-dPDT group than in the placebo-daylight group (RR: 0.18; 95% CI: 0.08–0.41). The number needed to treat (NNT) to have a benefit from the experimental therapy was 1 (95% CI: 1.11–1.78).
After randomization, one patient was eligible according to her global facial photodamage, but he did not meet the age criteria (she was 33 years-old). However, she completed the study and was analyzed accordingly to the group in which she was allocated.
Also, time between the second and the third session was extended more than a month due to administrative reasons that caused a delay in placebo shipping.
Secondary outcomesSignificant differences were also found in specific photodamage variables (Table 2).
Photodamage severity scores.
| Photodamage severity Scores | Placebo+daylight | MAL+daylight | p value | RR of failure with 95% CIa |
|---|---|---|---|---|
| Global photodamage scores | ||||
| Failure | 27 (90%) | 5 (17%) | 0.000 | 0.18 (0.08–0.41) |
| Improvement | 2 (7%) | 15 (50%) | ||
| Success | 1 (3%) | 10 (33%) | ||
| Specific photodamage severity scores | ||||
| Fine lines | ||||
| Failure | 27 (90%) | 6 (20%) | 0.000 | 0.22 (0.10–0.45) |
| Improvement | 2 (7%) | 13 (43%) | ||
| Success | 1 (3%) | 11 (37%) | ||
| Mottled pigmentation | ||||
| Failure | 23 (77%) | 7 (23%) | 0.000 | 0.30 (0.15–0.59) |
| Improvement | 4 (13%) | 18 (60%) | ||
| Success | 3 (10%) | 5 (17%) | ||
| Sallowness | ||||
| Failure | 25 (83%) | 5 (17%) | 0.000 | 0.20 (0.08–0.45) |
| Improvement | 2 (7%) | 9 (30%) | ||
| Success | 3 (10%) | 16 (53%) | ||
| Tactile roughness | ||||
| Failure | 25 (83%) | 5 (17%) | 0.000 | 0.20 (0.08–0.45) |
| Improvement | 2 (7%) | 6 (20%) | ||
| Success | 3 (10%) | 19 (63%) | ||
| Coarse lines | ||||
| Failure | 27 (90%) | 9 (30%) | 0.000 | 0.33 (0.19–0.58) |
| Improvement | 3 (10%) | 15 (50%) | ||
| Success | 0 (0%) | 6 (20%) | ||
| Erythema | ||||
| Failure | 26 (86%) | 6 (20%) | 0.000 | 0.23 (0.11–0.47) |
| Improvement | 2 (7%) | 16 (53%) | ||
| Success | 2 (7%) | 8 (27%) | ||
Oozing, edema and vesiculation were not present in any group one week after each session (Table 3). Erythema and desquamation were significantly different in all sessions when both groups were compared, whereas pigmentation was statistically different only after the last session (Table 3).
Pain scores and effects after 1 week of all sessions.
| Other secondary outcomes | Placebo+daylight (mean(median)) | MAL+daylight (mean(median)) | p value | 95% CIa |
|---|---|---|---|---|
| Pain VAS score after session 1 (0–10) | 0.50 (0) | 0.70 (0) | N.S.b | −0.51 to 0.91 |
| Pain VAS score after session 2 (0–10) | 0.23 (0) | 1.63 (0) | 0.00 | 0.41 to 2.39 |
| Pain VAS score after session 3 (0–10) | 0.80 (0) | 1.10 (0) | N.S.b | −0.69 to 1.29 |
Pain VAS scores after session 1 and 3 were not significantly different between the two groups, whereas they were found to be significantly different in session 2 (Table 4).
Secondary effects scores after 1 week of all sessions (Z test for proportions comparison).
| Other secondary outcomes | Placebo (mean(median)) n (%) | MAL+daylight (mean(median)) n (%) | p value for individual percentage differences | p value (Z test for global proportions comparisons) | 95% CIa |
|---|---|---|---|---|---|
| Reaction 1 week after session 1 | |||||
| Oozing | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Erythema | |||||
| 0 | 30 (100%) | 15 (50%) | <0.05 | 0.00 | 28–71% |
| 1 | 0 (0%) | 13 (43%) | <0.05 | −64% to −22% | |
| 2 | 0 (0%) | 2 (7%) | >0.05 | −18% to 5% | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Edema | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Desquamation | |||||
| 0 | 30 (100%) | 9 (30%) | <0.05 | 0.00 | 50–89% |
| 1 | 0 (0%) | 14 (47%) | <0.05 | −67% to −25% | |
| 2 | 0 (0%) | 7 (23%) | <0.05 | −41% to −4% | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Pigmentation | |||||
| 0 | 28 (93%) | 30 (100%) | >0.05 | N.S.b | −18% to 5% |
| 1 | 2 (7%) | 0 (0%) | >0.05 | −5% to 18% | |
| 2 | 0 (0%) | 0 (0%) | – | – | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Vesiculation | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Reaction 1 week after session 2 | |||||
| Oozing | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Erythema | |||||
| 0 | 29 (97%) | 12 (40%) | <0.05 | 0.00 | 34–78% |
| 1 | 1 (3%) | 18 (60%) | <0.05 | −78% to −34% | |
| 2 | 0 (0%) | 0 (0%) | – | – | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Edema | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Desquamation | |||||
| 0 | 30 (100%) | 8 (27%) | <0.05 | 0.00 | 54–92% |
| 1 | 0 (0%) | 19 (63%) | <0.05 | −83% to −42% | |
| 2 | 0 (0%) | 3 (10%) | >0.05 | −24% to 4% | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Pigmentation | |||||
| 0 | 30 (100%) | 29 (97%) | >0.05 | N.S.b | −6% to 13% |
| 1 | 0 (0%) | 1 (3%) | >0.05 | −13% to 6% | |
| 2 | 0 (0%) | 0 (0%) | – | – | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Vesiculation | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Reaction 1 week after session 3 | |||||
| Oozing | |||||
| 0 | 30 (100%) | 30 (100%) | * | * | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Erythema | |||||
| 0 | 30 (100%) | 19 (63%) | <0.05 | 0.00 | 16–57% |
| 1 | 0 (0%) | 11 (37%) | <0.05 | −57% to −16% | |
| 2 | 0 (0%) | 0 (0%) | – | – | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Edema | |||||
| 0 | 30 (100%) | 30 (100%) | * | – | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
| Desquamation | |||||
| 0 | 27 (90%) | 10 (33%) | <0.05 | 0.00 | 33–80% |
| 1 | 3 (10%) | 17 (57%) | <0.05 | −70% to −22% | |
| 2 | 0 (0%) | 3 (10%) | >0.05 | −24–4% | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Pigmentation | |||||
| 0 | 30 (100%) | 24 (80%) | <0.05 | 0.02 | 2–37% |
| 1 | 0 (0%) | 6 (20%) | <0.05 | −37% to −2% | |
| 2 | 0 (0%) | 0 (0%) | – | – | |
| 3 | 0 (0%) | 0 (0%) | – | – | |
| Vesiculation | |||||
| 0 | 30 (100%) | 30 (100%) | * | – | – |
| 1 | 0 (0%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 0 (0%) | 0 (0%) | |||
The majority of Skindex-29 scores showed non-statistical differences when baseline/after treatment scores were compared (p>0.05). However, individual scores of the MAL group in item 5 (My skin condition affects my social life) and item 14 (I tend to do things by myself because of my skin condition) showed statistical significant differences (p<0.05).
Overall, mean outside temperature during sessions was 28.60°C. All patients sun-exposed for 120min. Mean illuminance in each session varied from 82,478.75 through 72,528.56 and 70,419.1736lx (sessions 1, 2 and 3, respectively). Mean solar irradiance (W/m2) during the first, second and third sessions was 480.82, 430.07 and 435.84, respectively.
Post hoc analysisThere was an imbalance between both groups in gender (more women than men) and just 1 patient in skin-phototype-I, it was not possible to evaluate effect interactions in these subgroups. However, when controlling only for skin phototype-II and III, a statistical significant association was found between the product received, and facial photodamage improvement (OR: 0.09; 95% CI: 0.01–0.81; p=0.03), whereas no statistical significant association was found between the intervention and the placebo group when irradiance was tested (OR: 1.00; 95% CI: 0.99–1.00; p=0.39). No association was found neither between age (OR: 1.05; 95% CI: 0.96–1.15; p=0.24) and treatment response, nor between facial photodamage improvement and time lapse between sessions (OR: 1.00; 95% CI: 0.95–1.05; p=0.92)
Also in the post hoc evaluation of subgroups interaction (excluding men and skin phototype-I), we did not find statistical significance of this relation (OR: 1.28; 95% CI: 0.23–7.17; p=0.77).
Adverse events (AE)Two patients in the MAL group had serious events not related to the product. Another two patients (belonging to the MAL group) had non-serious adverse events which corresponded to a recurrence of herpes simplex related to sun-exposure (not previously reported by the patient) and a stressful situation. Another patient had a reaction to diacerein which was prescribed by her physician one week after the third session. Also, none of the patients in the placebo group presented with any AE.
DiscussionThis study showed that dPDT was unpainful, safe and effective in the treatment of facial photodamage. The size of the effect obtained by dPDT was so high that just one patient has to be treated to have a benefit with this intervention. Although no previous published study has evaluated the benefits of dPDT in photodamage as main outcome, this study supports what has been observed in other studies in which skin photodamage signs improve after cPDT in the treatment of AKs or facial photodamage.8,20,21
The median age of individuals in this trial was 60 years, which is in agreement with the age of most people with photodamage signs in any country, although such signs could present earlier or more pronounced in Equator zones or in highly sun exposed people such as farmers or outdoor workers.22,23
In MAL-treated patients, a higher effect was found in sallowness and tactile roughness, although other photodamage signs (i.e., fine lines, mottled pigmentation erythema, coarse lines), also improved. These findings are in agreement with reported effects obtained with cPDT, except for erythema.8 Such results could be explained by the light source used, as conventional PDT with red-light has been reported to induce more erythema.16,24 Regarding mottled pigmentation, Dover's photodamage scale does not differentiate pigmentation due to seborrheic keratosis, lentigos, melasma or pigmented actinic keratosis. As we did not include these specific outcomes in efficacy assessment, we hypothesize that facial pigmentation improvement could have been obtained by MAL's proved effect in pigmented AKs and perhaps in lentigos.
Pain during illumination in cPDT is the main unwanted effect of the procedure.8,15,25 In this study, facial photodamage treatment resulted in overall low pain scores which is a very relevant finding as pain is the main drawback of all other available facial photodamage therapy modalities or rejuvenating procedures such as chemical peelings, IPL and lasers.7,26,27
Early secondary effects after PDT sessions have been extensively studied.8,15,16,24 In this study only erythema and desquamation were found to be significantly different in all sessions. Such agreements or disagreements with published literature could be explained by the time at which such outcomes are assessed, as the majority of studies have evaluated these endpoints during the first 3 days when inflammatory signs are more frequent.15,16,24 When early pigmentation was evaluated, we found that it increased gradually from session to session resulting in significant differences after the third session. This could be explained by transient post-inflammatory pigmentation or a cumulative pigmentation due to ethnic skin responses to sun exposure, a fact that has already been reported in a similar population treated with cPDT.8 Importantly, mottled pigmentation improved in the MAL group when compared to placebo, one month after the third session.
When irradiance was analyzed, no statistical differences were found between both groups. This finding could be explained by a high mean solar irradiance during the daylight sessions of our study, which has exceeded the mean irradiance that has proved to induce a clinical benefit (305 Watts/m2).17,28,29
In this study we quantified QOL with the skindex-29 scale, which is the only instrument properly validated and adapted in Colombia.30 Although the majority of item scores did not change after therapy, this could be explained by a whole scale failure and lack of sensitiveness to detect baseline or final impact in facial photodamage. However, significant findings and changes in 2 function items of the scale (pre-/post-treatment) suggest that some important QOL features are impaired in skin photodamage and they in fact could be improved by dPDT. Even though no previous published studies regarding this issue have been found.
In our study, adverse events were more frequent in MAL-dPDT group. However, although such events were not related directly with the study product, the practitioner has to be cautious regarding sun exposure and consider herpes simplex prophylaxis in patients with a history of recurrent episodes.
The strength of this study lies on the double-blind placebo-controlled randomized design chosen, and the methodological rigor of trial performance and monitoring, which followed strict good clinical practice regulations. This is important as very few well designed trials exists to evaluate the efficacy of treatments for photodamage.
Limitations of our trial include the inability of this study for generalizing findings in men and in patients with skin phototype-I and the lack of the use of a validated and reliable scale for photodamage assessment, which could have led to more objective and quantitative measures of therapy effects. Also, in this study, an imbalance in important baseline characteristics (gender, skin phototype and global photodamage score) was found, having more men and lighter skin patients in the placebo group whereas skin photodamage was increased in MAL-group individuals. Nevertheless, after performing post hoc sub-group analysis and covariate adjustment, only skin phototype was found to have a role in the main outcome and no relationship was found with age. This finding is very important as we are not aware of any published study reporting such results. However, we have to bear in mind that post hoc subgroup analyses and non a priori covariate adjustment are both merely exploratory.
In conclusion, dPDT with MAL was painless, effective and safe for the treatment of global facial photodamage when compared with placebo. This therapy was also useful for the treatment of fine lines, coarse lines, tactile roughness, mottled pigmentation, sallowness, and erythema. Also, in high risk patients, herpes simplex prophylaxis should be considered before sessions. Results obtained are encouraging as dPDT with MAL not only is effective, but also easy to apply and straightforward to be monitored by any dermatologist. Finally, larger studies such as phase III designs with a more objective quantification of photodamage signs are required in order to confirm efficacy but also to evaluate long-term safety and to determine subgroup differences for men and certain skin phototypes.
Conflict of interestMethyl aminolevulinate (Metvix®), matching placebo and Cetaphil Dermacontrol SPF30® were provided free of charge by its manufacturer (Galderma SA Laboratories), but the pharmaceutical lab was not involved in trial design, and did not participate in analysis of the data or in the preparation of the final manuscript or in the publication of results.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
This work was supported by the Group of Investigative Dermatology-GRID and by the Fundacion Dermabase.
We thank Dr. Jhon Jairo Zuleta for his critical review of the manuscript. We also thank all the patients who participated in this study and the IPS Universitaria for its staff willingness to assist in the development of the trial.
We are also grateful to Galderma for providing the sunscreen, the product and the placebo free of charge and to Cristian Santa (Statistician -Universidad Nacional, Medellin, Colombia) for the generation of the randomization sequence.
We are very grateful to Ana Cristina Cardona, (professional nurse) for her valuable help in the development and execution of the trial and we also thank Veronica Ruiz (Dermatology Resident) for her trial assistance.
Dr. Gloria Sanclemente is a PhD Candidate at the Universitat Autonoma de Barcelona, Spain (Departament de Pediatria, d’Obstetrícia i Ginecologia i de Medicina Preventiva).