Cutaneous melanoma (hereinafter, melanoma) is the cause of most deaths due to skin cancer. The different registers throughout the world show sustained growth in the incidence of melanoma since the Second World War.1 This increase has been attributed to social changes in behavior regarding the sun, changing from avoiding exposure to the sun to actively seeking a suntan, marked not only by the beauty standards of the 21st century2 but also by the belief in the healthy benefits of exposure to natural or artificial ultraviolet radiation, even in children, which has spread increasingly since the end of the 19th century.3–5
In terms of mortality, a very marked increase has also been observed from the middle of the 20th century up until the 1990s, when a stabilization and even a drop in mortality due to melanoma was observed in most countries, such as Australia6 and the United States.7 Recent studies, however, continue to show a constant increase in mortality.8
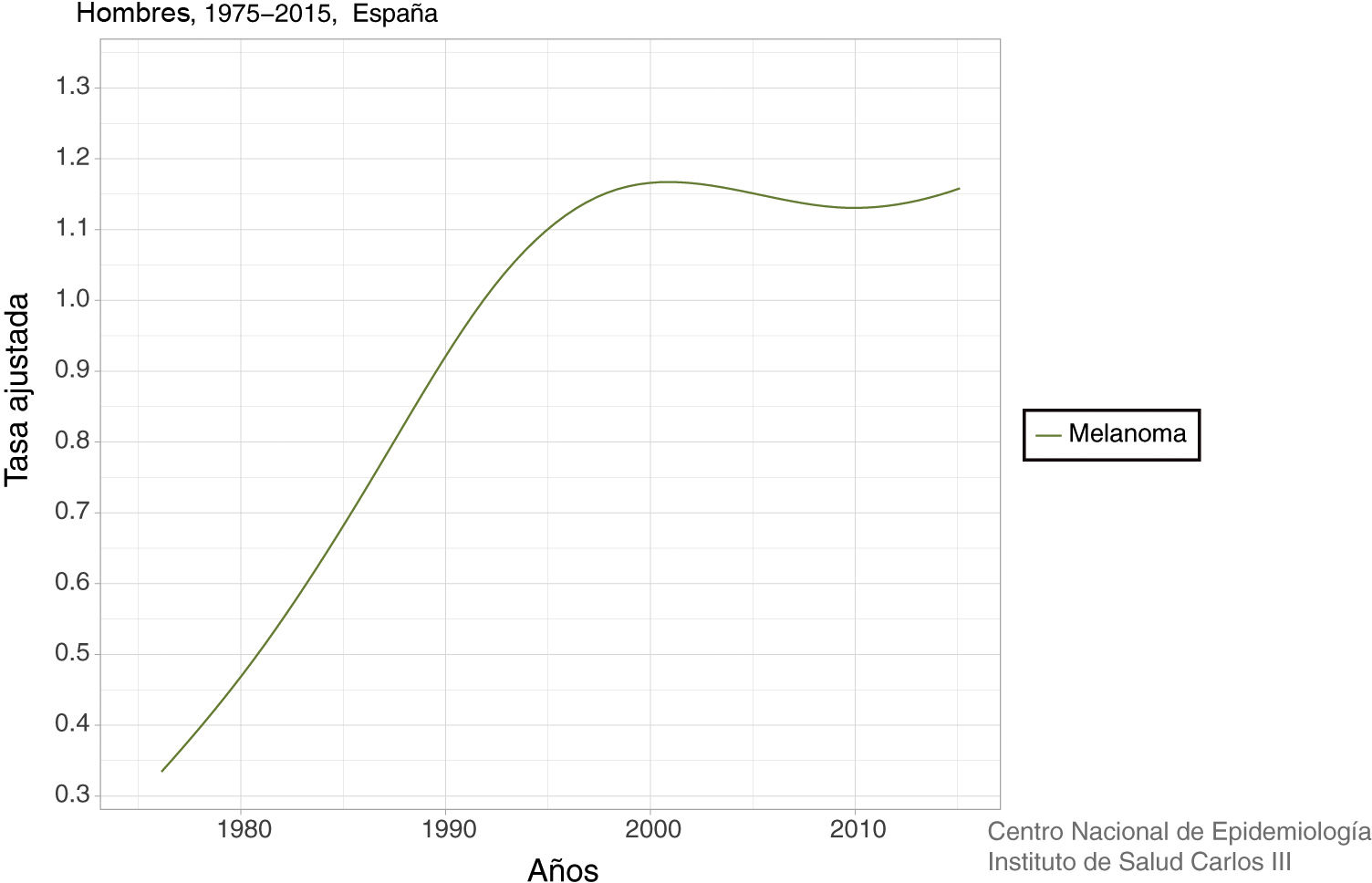
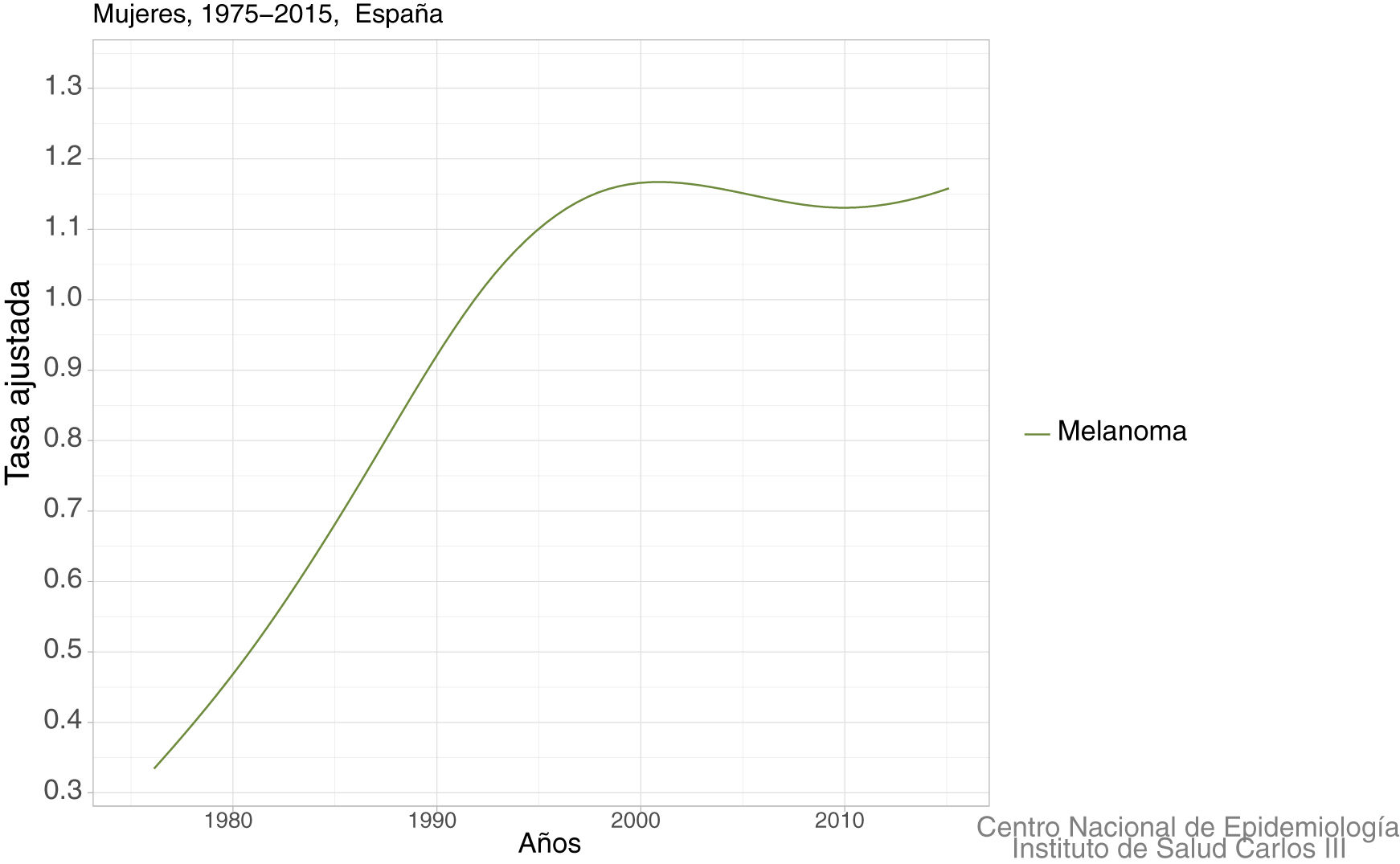
In Spain, a recent systematic review estimated an overall crude incidence of 8.82 cases per 100,000 people per year, with an overall crude mortality rate of 2.17 cases per 100,000 people per year.9 Data on mortality for Spain, adjusted for the world population, can be viewed on the Ariadna Interactive Epidemiology Information Server, which is part of the Spanish National Institute of Statistics.10Fig. 1 shows the adjusted mortality rate, which shows a 4-fold increase until the 1990s for men and a 3-fold increase for women in the same period. Since then, the rate has remained stable for women and has even increased slightly for men (Fig. 2).
In the current context, when awareness of the harmful effects of ultraviolet radiation is high, when there is a clear increase in diagnosis of increasingly thin melanomas, and when we have better treatment for advanced stages, a reasonable question arises: why, then, is mortality due to melanoma not falling?
The diagnosis of thick melanomas has remained stable or increased.
Available epidemiological data show that, in Europe, there has been a clear increase in melanomas diagnosed in early stages (in situ and <1 mm). However, diagnosis of melanomas of intermediate thickness (1-4 mm) and thick melanomas (>4 mm) has increased in practically all countries in recent years,11 despite efforts aimed at early diagnosis of melanoma. This ongoing problem of thick lesions is well known, not only in Spain12 but also in other parts of the world.13
The epidemiologic trends of melanoma led Lipsker et al.14 in 2007 to propose the existence of 3 types of melanoma: type I or thick, fast-growing melanomas with a stable incidence and random distribution on the body, with little relationship to UV exposure, and which tend to affect people with few nevi and with no other risk factors; type II or intermediate-growth melanomas, with a distribution in areas with intermittent exposure to the sun, such as the torso and extremities, more frequent in young people, and with greater prevalence of BRAF mutations; and type III or slow-growth melamomas located on areas chronically exposed to the sun, such as the head and neck in elderly people. Different histopathology, confocal-microscopy and, particularly, gene-expression studies support and justify this classification.15 One hypothesis suggests that type I melanomas originate in melanocytes located in the dermis. For this reason, they are lesions that already have a considerable thickness when they acquire sufficient importance as to be diagnosed.
The ageing of the population is a key element of the overall mortality rate.
The impact on mortality, or at least a partial explanation of it, is to be found in the recent study by Cayuela et al. on the trend of mortality due to melanoma by age strata in Spain.16 Using a jointpoint regression model, this study corroborated an increase in mortality up until the 1990s for all age strata. From then until the present, the most relevant data show a drop in mortality in the age group under 50 years, stabilization in the 50-59 years age group, and an increase for those over 69 years of age (especially men).
This increase in mortality due to melanoma in elderly patients is not limited to Spain. Autier et al.17 carried out a larger study using a similar methodology and the database of the World Health Organization, from 1955 to 2010, in which they grouped countries by continent and, in the case of Europe, by region. In general terms, the data are very similar, with a drop in mortality for those under 50 years of age, stabilization for the 50-69 years age group, and an increase for those over 70 years of age. However, the methodology of this study has made it possible to estimate mortality rates by birth generation (cohort effect).
This makes it possible to calculate the cumulative risk of dying from melanoma throughout life and the peak risk of dying from melanoma based on the year and region of birth. In summary, for patients from northern Europe, a peak incidence was reached in those born at the beginning of the 1940s. This was very similar to North America and Oceania. This peak is found with a little delay in western Europe and, in the 1950s, for central Europe and the United Kingdom and Ireland. For all those born after the different annual peaks in the different regions, the risk has fallen gradually to approach the risk that these regions had at the beginning of the 20th century.
But what about the estimation for southern Europe, where we live? The data show that this peak has not yet been reached. Thus, it is to be expected that we are continuing to experience an increase in mortality due to melanoma, especially in the elderly population, whereas this will tend to be rare in the young population under the age of 50 years, a trend in line with an increase in the incidence of melanoma, which is expected to be more pronounced in the elderly population.8
This makes it necessary to achieve a better understanding of the behavior and treatment of melanoma in the elderly, a topic that has been thoroughly reviewed in this very journal.18 In general, melanoma in the elderly is diagnosed with a delay that will lead to greater thickness and a more advanced stage, which will contribute to maintaining the overall mortality rate.
A delay of more than 2 months in visiting the doctor has been shown to be associated with a low or inexistent awareness of the disease.19 Thus, we sometimes see patients who have delayed their medical visit due to the strange and mistaken belief that excising a benign lesion may lead to the same lesion becoming malignant in the future.20
Other factors exist, however, that we may call tumor-dependent, which will complicate the task of early diagnosis in elderly patients. Amelanotic and fast-growing melanomas are more frequent in this group.21,22 The association of rapid-growth tumors and delays in visiting the doctor lead to tumors of greater thickness at the time of diagnosis. It has been estimated that, for rapid-growth melanomas, metastatic capacity occurs in the initial weeks after the patient notices the lesion.23 These melanomas are easily missed by educational programs due to this rapid growth.
Thus, in the current context, when we have one of the highest (and increasing) life expectancies in the world, it is easy to predict that it will not be easy to reduce the overall mortality rate due to melanoma.
It is necessary to characterize this type of thick melanoma, as far as possible, in this target population in order to determine the most appropriate tools for prevention and early diagnosis. Time will tell whenther this is a vision or an impossible mission, as other authors argue.15
Please cite this article as: Tejera-Vaquerizo A, Boada A, Nagore E. ¿Por qué no disminuye la mortalidad por melanoma cutáneo? Actas Dermosifiliogr. 2020;111:450–452.