Herpes zoster is caused by reactivation of the varicella zoster virus, which is characterized by a generally localized and painful vesicular skin rash. Its incidence has increased in recent decades. The most frequent complication is postherpetic neuralgia, which affects around 18% of patients with herpes zoster and may cause disabling pain. The incidence of both conditions clearly increases with age. Postherpetic neuralgia affects more than 30% of persons with herpes zoster aged >79 years. The attenuated varicella zoster virus vaccine (aVZV) has an efficacy of 51% for prevention of herpes zoster; this efficacy decreases with age. The vaccine is contraindicated in immunocompromised patients and approved by the United States Food and Drug Administration (FDA) for this indication in immunocompetent patients aged ≥50 years.
The FDA recently approved a new recombinant vaccine for treatment of herpes zoster (rVZV) (Shingrix, GlaxoSmithKline). rVZV contains recombinant glycoprotein E of the varicella zoster virus, together with the adjuvant AS01B, and is administered in 2 subcutaneous injections separated by 2–6 months (Table 1). The efficacy of rVZV was evaluated in 2 phase 3 randomized, placebo-controlled clinical trials (ZOE-501 and ZOE-702). Median follow-up was longer than 3 years in both trials. ZOE-50 included 15 411 patients aged ≥50 years and reported 6 cases of herpes zoster in the vaccinated group compared with 210 in the placebo group. Overall efficacy in the prevention of herpes zoster was 97.2% (95% CI, 93.7–99.0%; P<.001).1 ZOE-70 included 13 900 patients aged ≥70 years. Efficacy was 89.8% (95% CI, 84.2–93.7%; P<.001) for prevention of herpes zoster and 88.8% (95% CI, 68.7–97.1%; P<.001) for prevention of postherpetic neuralgia.2 A pooled analysis of both studies showed that efficacy was 93.7% (95% CI, 59.5–99.9%) for the prevention of post–herpes zoster complications other than postherpetic neuralgia, i.e., disseminated herpes zoster, secondary vasculitis, encephalic vascular accident, and ophthalmic, neurologic, and visceral disease.3 In both studies, the frequency of adverse events was similar in the vaccination and placebo groups. The most frequent adverse events were injection site pain, myalgia, and fatigue.
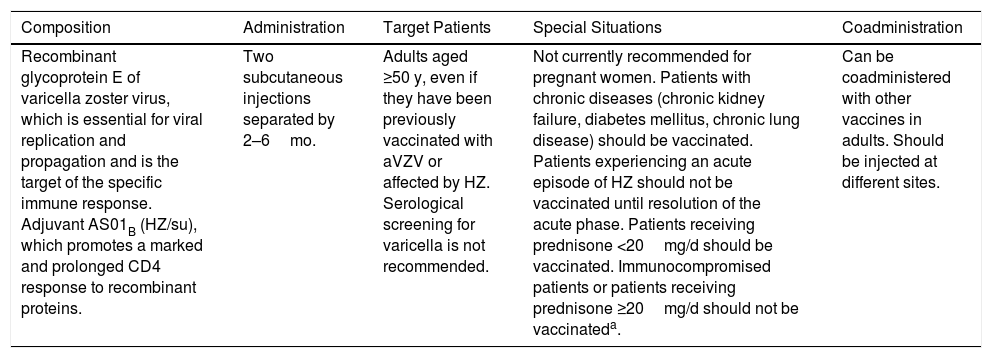
Characteristics and Indications of the Recombinant Herpes Zoster Vaccine (Shingrix).
| Composition | Administration | Target Patients | Special Situations | Coadministration |
|---|---|---|---|---|
| Recombinant glycoprotein E of varicella zoster virus, which is essential for viral replication and propagation and is the target of the specific immune response. Adjuvant AS01B (HZ/su), which promotes a marked and prolonged CD4 response to recombinant proteins. | Two subcutaneous injections separated by 2–6mo. | Adults aged ≥50 y, even if they have been previously vaccinated with aVZV or affected by HZ. Serological screening for varicella is not recommended. | Not currently recommended for pregnant women. Patients with chronic diseases (chronic kidney failure, diabetes mellitus, chronic lung disease) should be vaccinated. Patients experiencing an acute episode of HZ should not be vaccinated until resolution of the acute phase. Patients receiving prednisone <20mg/d should be vaccinated. Immunocompromised patients or patients receiving prednisone ≥20mg/d should not be vaccinateda. | Can be coadministered with other vaccines in adults. Should be injected at different sites. |
Abbreviations: HZ, herpes zoster; HZ/su, HZ/subunit; aVZV, attenuated varicella zoster vaccine.
rVZV has proven more successful than aVZV for prevention of herpes zoster and postherpetic neuralgia. Furthermore, the cost-effectiveness analysis favored rVZV over aVZV.4 Consequently, the Advisory Committee on Immunization Practices in the United States recently recommended rVZV for immunocompetent patients aged ≥50 years, even in those previously vaccinated with aVZV and those who have had herpes zoster.5 Evidence remains insufficient to recommend rVZV in immunocompetent patients or patients who are receiving immunosuppressive treatment with prednisone ≥20 mg/d, although it could, in theory, be administered.
rVZV is an efficacious and safe alternative for prevention of herpes zoster and postherpetic neuralgia and should be recommended in adults. It could thus help to reduce the morbidity associated with these conditions.
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Giavedoni P. FR-Nueva vacuna recombinante para la prevención del herpes zóster. Actas Dermosifiliogr. 2020;111:67–68.