Hidradenitis suppurativa is a chronic and recurrent inflammatory disease that causes disfiguring lesions in areas rich in apocrine sweat glands.1
Prevalence is estimated at 1% to 4%, women are affected more often than men (at a ratio of 3:1), and onset is typically in the second or third decade of life.1,2
While its etiology and pathogenesis are largely unknown, hidradenitis suppurativa is considered a multifactorial disease in which the immune system plays a prominent role. Management of hidradenitis suppurativa should be tailored to lesion severity and distribution assessed according to the Hurley staging system.3 As no specific treatments are available, a wide range of therapeutic options are used with highly variable results. Antibiotics, corticosteroids, and retinoids may be helpful in the early stages and during exacerbations; in advanced or extensive forms of the disease, surgical resection of the affected tissue is imperative.4 Biologic agents, particularly tumor necrosis factor (TNF) inhibitors, have been proposed in recent years for recalcitrant forms of hidradenitis suppurativa. Experience with other biologic agents having different mechanisms of action, such as p40 inhibition, is anecdotal.
We report the case of a 50-year-old female smoker with moderate-to-severe hidradenitis suppurativa (Hurley stage II-III). The condition had been diagnosed at age 16 years and had worsened 25 years later. Recent treatments had included a course of isotretinoin and 4 surgical procedures that had involved the face and breasts.
In April 2009, as the condition had proved refractory to treatment, the patient started a new treatment with an 80-mg loading dose of adalimumab, followed by 40 mg every 14 days beginning one week later. Her condition remained stable for 1 year, after which the dosing interval was increased to 21 days. However, 6 months after this change the lesions had worsened and the 14-day regimen was reinstated, with the addition of prednisone for 1 month. Treatment was discontinued 6 months later owing to loss of efficacy (i.e. lesion recurrence) and adverse effects.
After 2 years of adalimumab, the patient was switched to infliximab 5 mg/kg at weeks 0, 2, and 6; however, this regimen was discontinued after 3 doses because the lesions worsened.
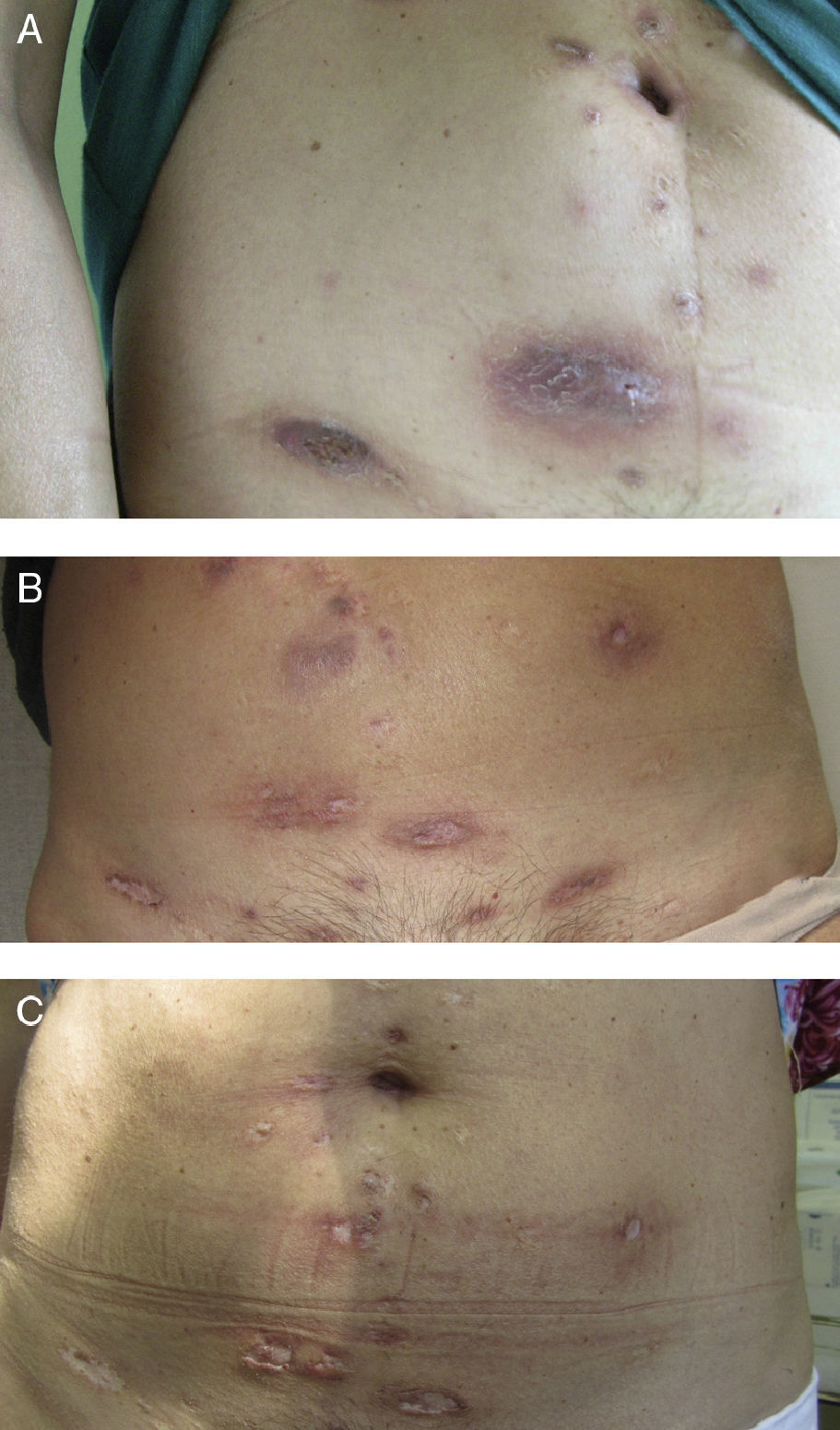
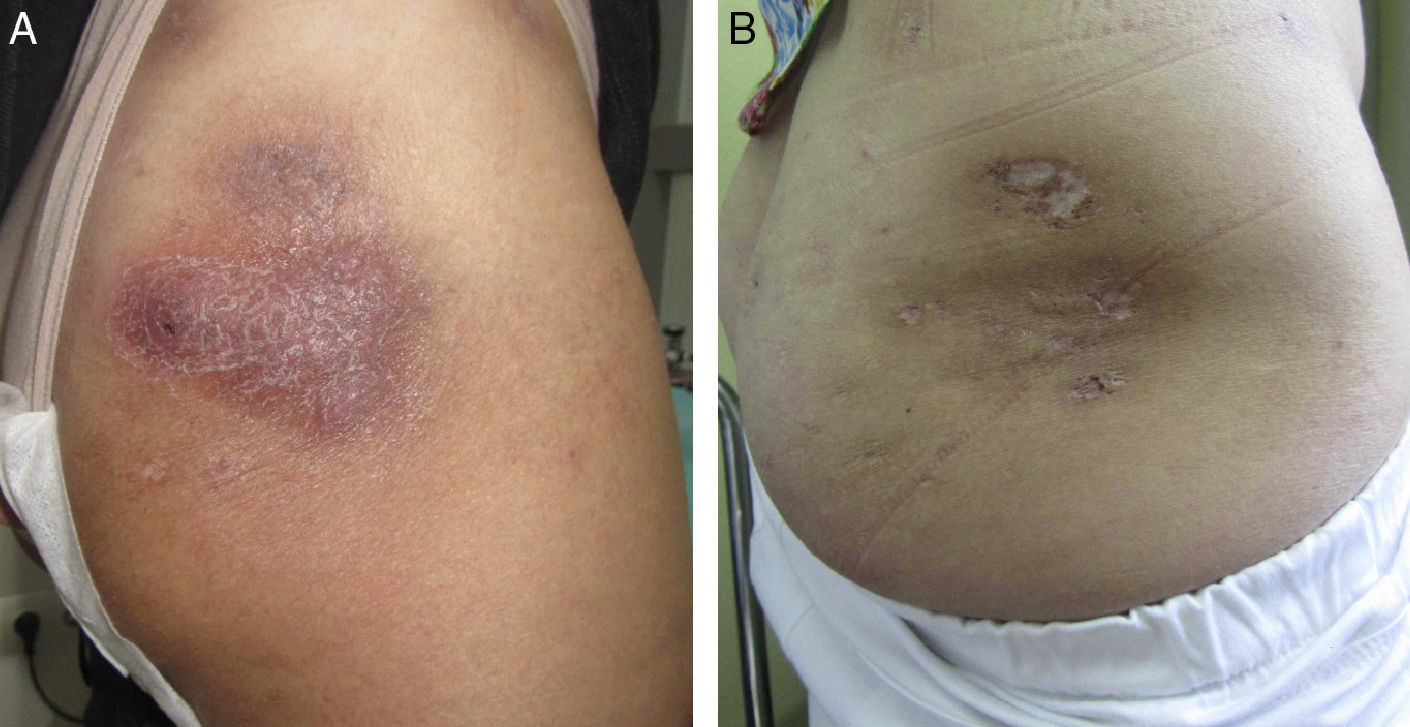
In October 2011, because of the severity of her lesions and the lack of other options, off-label treatment was proposed with 45 mg of subcutaneous ustekinumab at weeks 0 and 4, and at 12-week intervals thereafter, and hospital authorization was granted. Disease activity ceased to progress from the start of treatment, although 3 months passed before a clear improvement of the lesions was observed. After 8 months the disease was no longer active (Figs. 1 and 2). At the time of writing, 1½ years into treatment with ustekinumab, the patient has no active lesions and is tolerating therapy well. During the treatment period there were 2 exacerbations. Both were resolved with a 3-week course of antibiotics (amoxicillin-clavulanic acid at 500 mg/8 h in one case, and rifampicin at 300 mg/12 h in the other), plus prednisone.
Hidradenitis suppurativa is an orphan disease, not because its prevalence (1%-4%) is low, but because there are no therapies that produce sustained clinical remission, let alone any curative effect.
Recent studies in hidradenitis suppurativa have highlighted the role of the immune system and its proinflammatory action, including overexpression of interleukins 12 and 23 and of TNF.5,6
TNF inhibitors are a therapeutic option for advanced-stage patients who fail to respond to conventional treatment, but response, if achieved, appears to last only as long as treatment is continued. Because of this drawback, as well as their side effects and high cost, TNF inhibitors are relegated to second- or third-line treatment.7 Ustekinumab, a monoclonal antibody that blocks interleukins 12 and 23, is rarely used to treat hidradenitis suppurativa, and conclusive evidence on its efficacy is lacking.
In the case described, after the patient's condition had failed to respond well to conventional therapy or 2 different TNF inhibitors, we requested authorization for off-label use of ustekinumab, a drug indicated for moderate to severe psoriasis. Treatment was initiated once the authorization was received, and the patient remains clinically stable at the time of writing after 1½ years’ treatment.
A review of the literature shows that the experience with ustekinumab in hidradenitis suppurativa is anecdotal, with just one 3-case series in which response to treatment was uneven and 2 individual case reports of patients who had other associated inflammatory skin conditions (psoriasis and Behçet disease).8–10
The data presented suggest that ustekinumab could be a therapeutic option for treatment-refractory hidradenitis suppurativa.
Please cite this article as: Santos-Pérez MI, García-Rodicio S, del Olmo-Revuelto MA, Pozo-Román T. Ustekinumab en hidradenitis supurativa: a propósito de un caso. Actas Dermosifiliogr. 2014;105:720–722.