Locoregional lymph node ultrasound is not typically included in guidelines as part of the staging process prior to sentinel lymph node biopsy (SLNB). The objective of the present study was to make a clinical and economic analysis of lymph node ultrasound prior to SLNB.
Materials and methodsWe performed a retrospective study of 384 patients with clinical stage I-II primary melanoma who underwent locorregional lymph node ultrasound (with or without ultrasound-guided biopsy) prior to SLNB between 2004 and 2015. We evaluated the reliability and cost-effectiveness of the strategy.
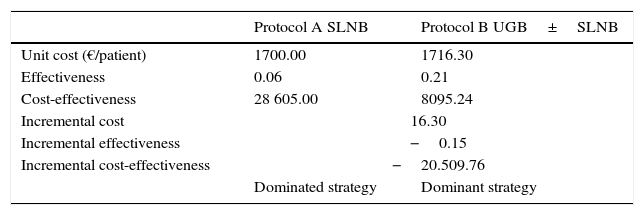
ResultsUse of locorregional lymph node ultrasound avoided SLNB in 23 patients (6%). Ultrasound had a sensitivity of 46% and specificity of 76% for the detection of metastatic lymph nodes that were not clinically palpable. False negatives were significantly more common in patients aged over 60 years and in tumors with a thickness of less than 2mm. The staging process using SLNB and ultrasound with ultrasound-guided biopsy produced an increase of €16.30 in the unit price. Our cost-effectiveness analysis identified the staging protocol with ultrasound and SLNB as the dominant strategy, with a lower cost-effectiveness ratio than the alternative, consisting of SLNB alone (8,095.24 vs €28,605.00).
ConclusionsUltrasound with ultrasound-guided biopsy for the diagnostic staging of melanoma prior to SLNB is a useful and cost-effective tool. This procedure does not substitute SLNB, though it does allow to avoid SLNB in a not insignificant proportion of patients.
La ecografía ganglionar locorregional como parte de la estadificación previa a la biopsia del ganglio centinela (BSGC) en pacientes con melanoma no se incluye habitualmente en las guías de manejo. El objetivo de este estudio es evaluarla desde la perspectiva clínica y económica.
Pacientes y métodosEstudio retrospectivo de 384 pacientes con melanoma primario de estadios clínicos I-II, en el periodo 2004-2015, a los que se les realizó una ecografía ganglionar locorregional (±biopsia ecodirigida) previa a la BSGC. Se evaluó la fiabilidad de la técnica y se realizó un análisis de su coste efectividad.
ResultadosEn 23 pacientes (6%) se pudo evitar la realización de la BSGC mediante la técnica. La ecografía mostró una sensibilidad del 46% y una especificidad del 76% para la detección de ganglios metastásicos clínicamente no palpables. Los falsos negativos fueron significativamente más frecuentes en pacientes mayores de 60 años y con tumores <2mm. El coste unitario del proceso de estadificación mediante BSGC con ecografía-biopsia ecodirigida generó un sobrecoste de 16,30 €. El análisis coste-efectividad identificó como estrategia dominante desde ese punto de vista el protocolo de estadificación con ecografía y BSGC, con una ratio coste-efectividad inferior a la de la alternativa consistente en BSGC (8.095,24 vs 28.605,00 €)
ConclusionesLa ecografía con biopsia ecodirigida como método diagnóstico de estadificación previo a la BSGC es una herramienta útil y coste-efectiva, que no sustituye a la BSGC, pero que permite en un porcentaje no desdeñable de casos evitar su realización.
Melanoma is a neoplasm that, despite its small size, has a relatively high ability to metastasize, occasionally early. Its incidence and mortality have increased in recent decades, especially in whites.1,2
When establishing the prognosis of a patient with cutaneous melanoma, the most important factors to be taken into account are presence of metastasis, type and number of affected organs, and—if these data are not available—the thickness of the tumor and the presence of ulceration.3-5 Since it was first introduced in 1992,6 sentinel lymph node biopsy (SLNB) has become the main locoregional staging technique, making it possible to provide an accurate prognosis in patients with clinical and radiological evidence of localized disease (clinical stages I and II of the American Joint Committee on Cancer). Although SLNB is not particularly invasive, it is not free of complications, especially local complications such as seroma, hematoma, and surgical wound infection and, albeit more rarely, lymphedema. Furthermore, the technique requires patients to receive general or spinal anesthesia, with all its concomitant risks.
Performing additional radiological tests continues to be controversial, as the low rate of detection does not justify the costs. The tests themselves are expensive and can reveal incidentalomas (findings not related to melanoma that require new tests for characterization). Testing also causes anxiety for patients. Nevertheless, it seems reasonable to use financially and clinically profitable techniques that obviate SLNB in cases where it is not necessary. Several studies have evaluated a series of procedures (traditional ultrasound, high-resolution ultrasound, ultrasound-guided fine-needle aspiration, contrast ultrasound, and positron-emission tomography/computed tomography) in staging before SLNB, with diverse results.7-17 However, few studies consider the cost-effectiveness of these procedures.18
The objective of the present study is to evaluate the role of ultrasound, including ultrasound-guided biopsy (UGB) when necessary, as a staging technique before SLNB. The study aims to determine the percentage of cases in which it is possible to avoid SLNB and the cost-effectiveness of this approach in our setting.
Material and MethodsWe designed a retrospective observational study based on data collected from our institutional melanoma database.
The study population comprised all patients with cutaneous melanoma who had undergone lymph node ultrasound before SLNB as part of their staging study between January 1, 2004 and December 31, 2015 at the Instituto Valenciano de Oncología, Valencia, Spain. Therefore, we excluded patients in whom lymph node metastasis had been detected during the physical examination (palpable enlarged nodes confirmed by histopathology). We also excluded patients with mucosal, uveal, and unknown primary melanomas, as well as those with insufficient information on the result of the lymph node biopsy, UGB, or SLNB (ie, patients whose sentinel node was not identified were excluded).
The study was approved by the local ethics committee.
At our center, SLNB is performed in patients with melanomas measuring more than 0.75mm and in patients with thinner melanomas and any of the following risk factors: ulceration, presence of at least 1 mitosis/mm2, microscopic satellite lesions/vascular invasion, regression greater than 50%, or incomplete excision with an affected deep margin (regression stopped being used as a selection criterion in January 2015 after it was shown to be of no value for predicting sentinel node involvement).19,20 Patients with primary cutaneous melanoma who fulfilled the criteria for SLNB were systematically evaluated beforehand as part of the staging study using B-flow and Doppler lymph node ultrasound. Ultrasound was performed by 2 experienced radiologists (CD and MG). Lymph nodes were classified as benign, indeterminate, or suspicious for melanoma according to standard criteria (see above).21
The ultrasound examination covered all possible drainage territories depending on the site. In the case of indeterminate or suspicious findings, an ultrasound-guided core-needle biopsy (Tru-cut) was performed. Histopathology of the biopsies performed included hematoxylin-eosin study and immunohistochemistry (S100 and melan-A). In patients whose histology work-up revealed melanoma cells, therapeutic lymph node dissection of the affected lymphatic basin was performed directly. The remaining patients underwent SLNB, followed by therapeutic lymph node dissection if melanoma cells were present. The technique used to evaluate sentinel nodes at our center has been reported in detail elsewhere.22
The study endpoints were as follows: (1) the percentage of cases in which SLNB was obviated based on core-needle biopsy and ultrasound; (2) the clinical yield of core-needle biopsy in indeterminate and suspicious malignant nodes (both together and separately); (3) false negatives in ultrasound; (4) the cost-effectiveness ratio of initial staging of patients with melanoma depending on whether or not regional lymph node ultrasound was performed before SLNB.
Statistical and Cost AnalysisWe used contingency tables and calculated the sensitivity, specificity, positive predictive value, and negative predictive value for the ability to detect metastasis in both SLNB and UGB. For purposes of the study, true positives were defined as indeterminate or positive ultrasound scans with histologic confirmation using either core-needle biopsy or SLNB.
False positives were defined as indeterminate and positive ultrasound scans without lymph node metastasis, both in core-needle biopsy and in SLNB.
True negatives were defined as normal ultrasound scans with negative SLNB results; false negatives were defined as normal ultrasound scans with positive SLNB results.
The statistical analysis was performed using SPSS 20.0 (IBM Corp).
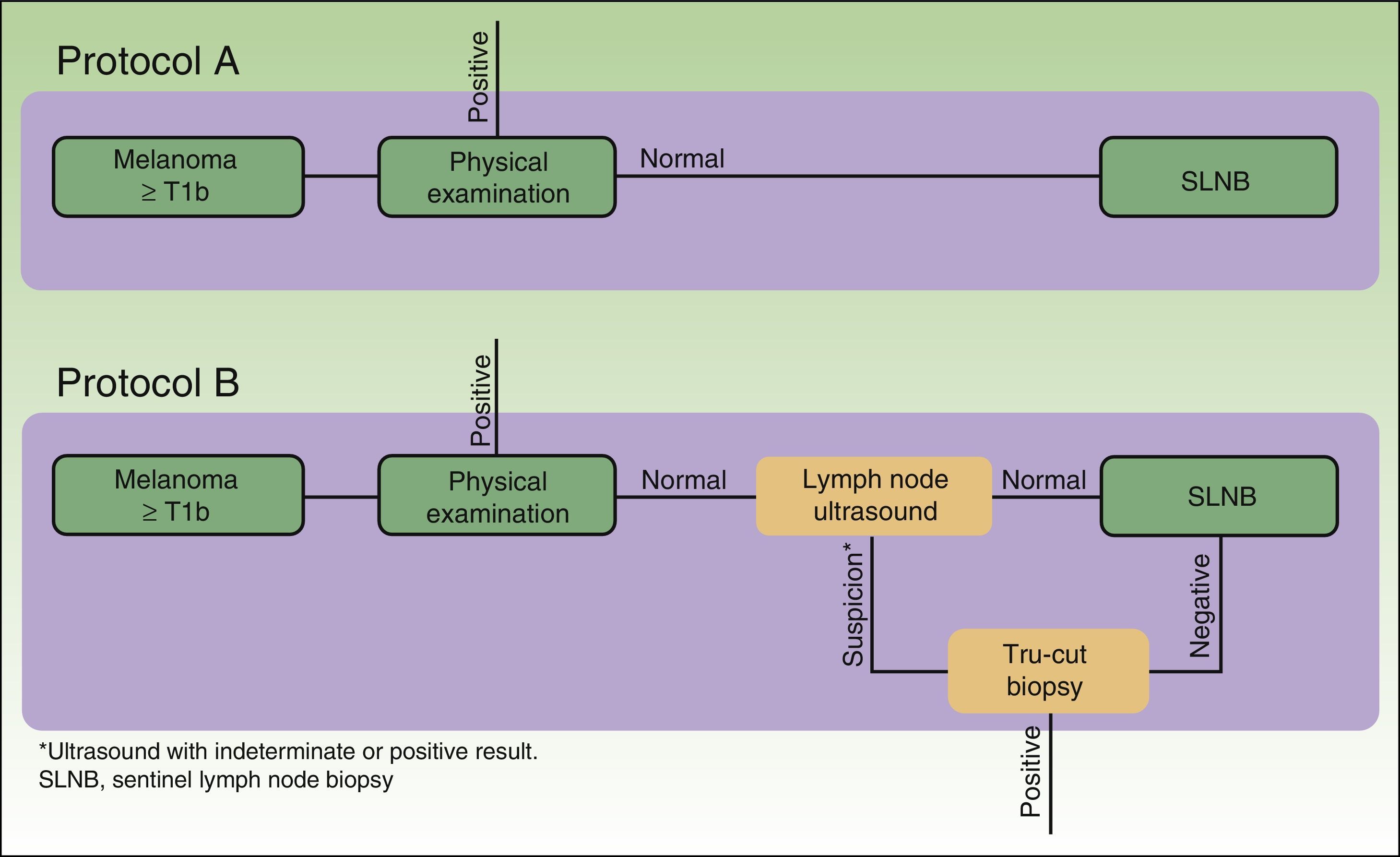
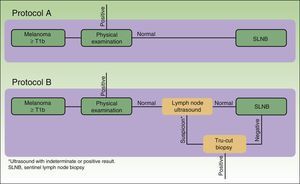
The cost analysis consisted of a cost-effectiveness study from the health service perspective. The study compared the efficiency of 2 theoretical protocols for staging patients with melanoma: one in which regional lymph node ultrasound was performed during the initial phase of staging and one in which it was not (Fig. 1).
In protocol A, lymph node ultrasound was not performed during initial staging; therefore, all selected patients underwent SLNB. In protocol B, all patients underwent regional lymph node ultrasound, as well as core-needle biopsy in cases where the results were indeterminate or positive. Patients in whom core-needle biopsy confirmed the presence of lymph node metastasis did not undergo SLNB (Fig. 1, selection).
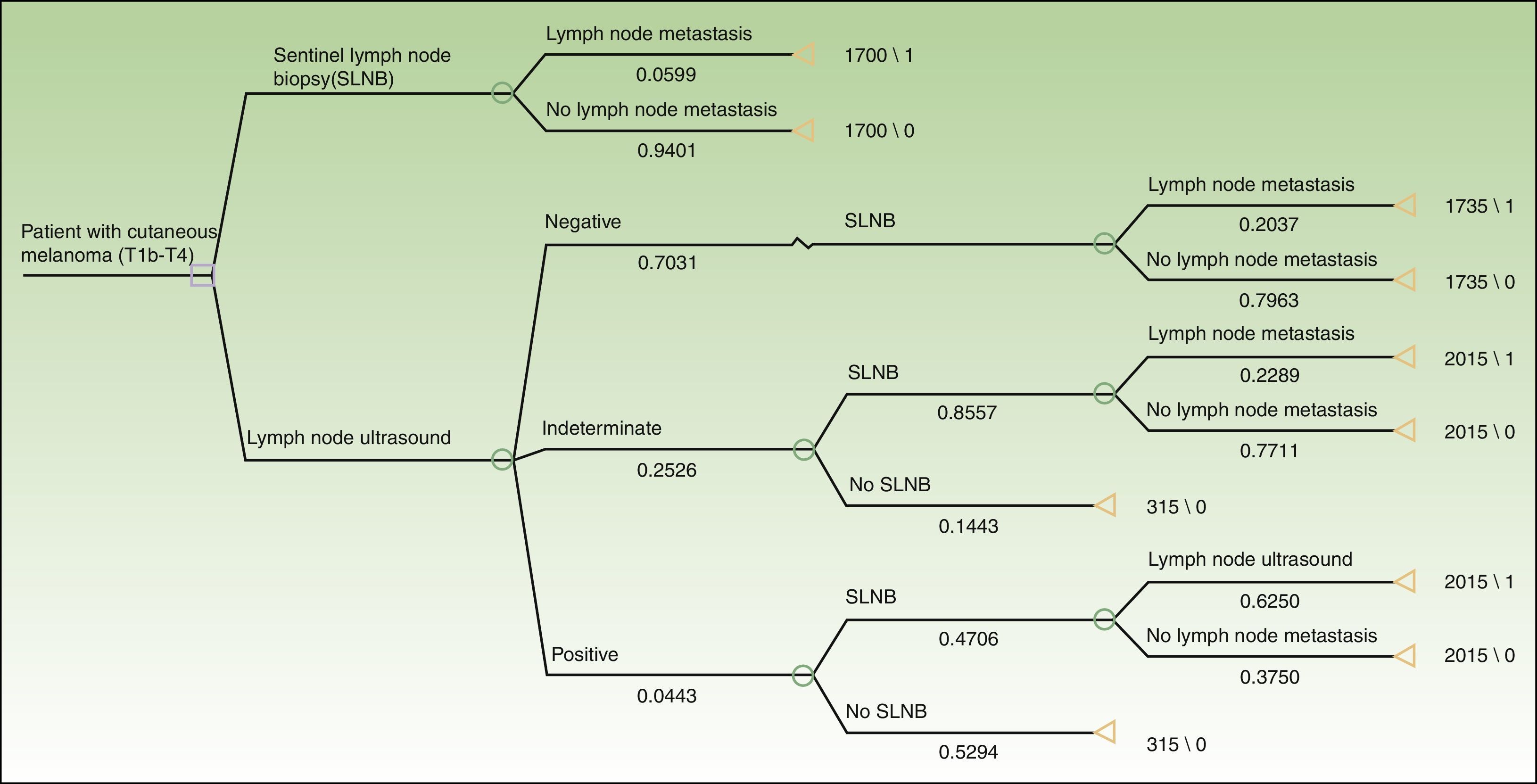
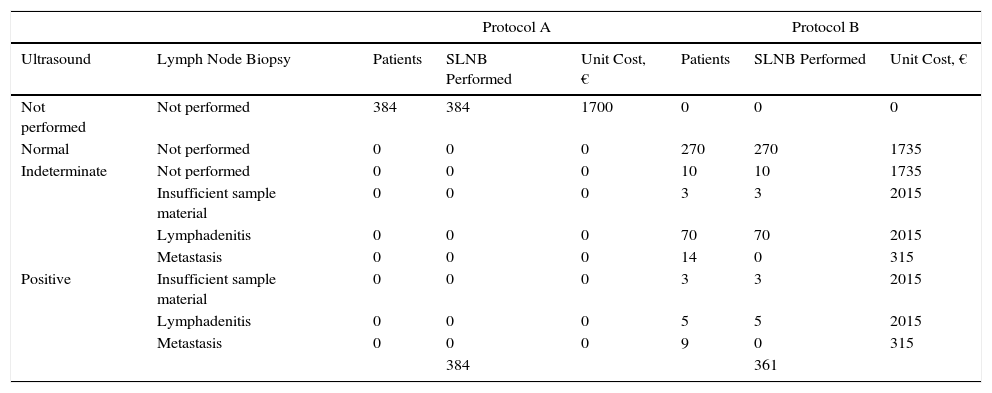
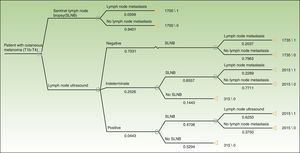
Based on the set of activities presented in Fig. 1, activity-based costing was applied to assign specific costs to the activities carried out in each of the protocols (lymph node ultrasound, core-needle biopsy, SLNB) (Fig. 1). Common activities and activities with foreseeably indistinguishable results in both protocols were excluded from the assignation of costs (physical examination). Costs were assigned to each activity by applying the rates in force in the health system where the study was performed, as follows: lymph node ultrasound, €35; core-needle biopsy, €280; SLNB, €1700. The prices set are the costs invoiced to third parties for these services and include direct and indirect costs arising from the activity of the center. These costs per activity and the frequency of events observed (lymph node metastasis identified) were used to calculate the unit costs (€/patient) (Table 1). These costs and the probability of events in each protocol were used to design a decision tree (TreeAge Pro 2016, TreeAge Software Inc.) to calculate the cost-effectiveness ratio for each protocol and to identify the dominant strategy (Fig. 2). The frequency of lymph node metastasis in each of the protocols compared was considered as a measure of effectiveness.
Procedures Performed, Events (Lymph Node Metastasis), and Costs of the Protocols Studied.
| Protocol A | Protocol B | ||||||
|---|---|---|---|---|---|---|---|
| Ultrasound | Lymph Node Biopsy | Patients | SLNB Performed | Unit Cost, € | Patients | SLNB Performed | Unit Cost, € |
| Not performed | Not performed | 384 | 384 | 1700 | 0 | 0 | 0 |
| Normal | Not performed | 0 | 0 | 0 | 270 | 270 | 1735 |
| Indeterminate | Not performed | 0 | 0 | 0 | 10 | 10 | 1735 |
| Insufficient sample material | 0 | 0 | 0 | 3 | 3 | 2015 | |
| Lymphadenitis | 0 | 0 | 0 | 70 | 70 | 2015 | |
| Metastasis | 0 | 0 | 0 | 14 | 0 | 315 | |
| Positive | Insufficient sample material | 0 | 0 | 0 | 3 | 3 | 2015 |
| Lymphadenitis | 0 | 0 | 0 | 5 | 5 | 2015 | |
| Metastasis | 0 | 0 | 0 | 9 | 0 | 315 | |
| 384 | 361 | ||||||
Abbreviation: SLNB, sentinel lymph node biopsy.
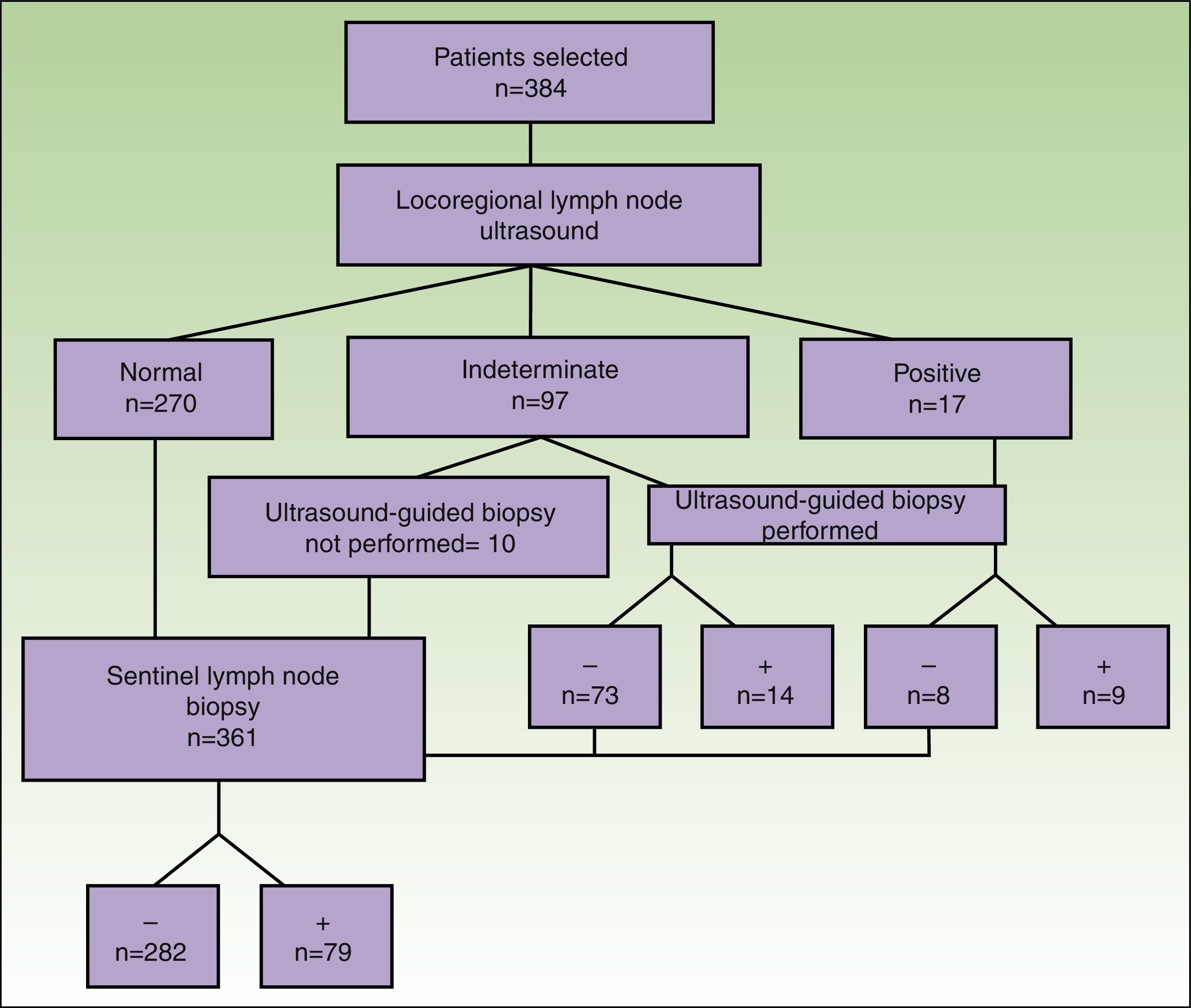
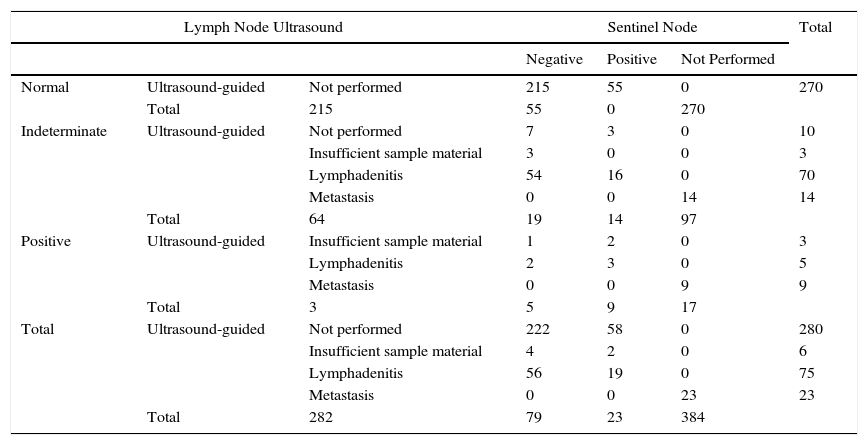
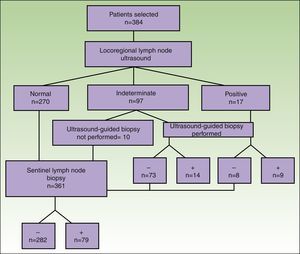
A total of 384 patients fulfilled the selection criteria and were included in the study (Fig. 3). The result of the ultrasound scan was normal in 270 patients, indeterminate in 97, and positive in 17 (Table 2).
Characteristics of Patients With Respect to Lymph Node Ultrasound, Ultrasound-Guided Biopsy, and the Result of SLNB.
| Lymph Node Ultrasound | Sentinel Node | Total | ||||
|---|---|---|---|---|---|---|
| Negative | Positive | Not Performed | ||||
| Normal | Ultrasound-guided | Not performed | 215 | 55 | 0 | 270 |
| Total | 215 | 55 | 0 | 270 | ||
| Indeterminate | Ultrasound-guided | Not performed | 7 | 3 | 0 | 10 |
| Insufficient sample material | 3 | 0 | 0 | 3 | ||
| Lymphadenitis | 54 | 16 | 0 | 70 | ||
| Metastasis | 0 | 0 | 14 | 14 | ||
| Total | 64 | 19 | 14 | 97 | ||
| Positive | Ultrasound-guided | Insufficient sample material | 1 | 2 | 0 | 3 |
| Lymphadenitis | 2 | 3 | 0 | 5 | ||
| Metastasis | 0 | 0 | 9 | 9 | ||
| Total | 3 | 5 | 9 | 17 | ||
| Total | Ultrasound-guided | Not performed | 222 | 58 | 0 | 280 |
| Insufficient sample material | 4 | 2 | 0 | 6 | ||
| Lymphadenitis | 56 | 19 | 0 | 75 | ||
| Metastasis | 0 | 0 | 23 | 23 | ||
| Total | 282 | 79 | 23 | 384 | ||
Consistent with the data recorded (Table 3), ultrasound as a technique for the detection of metastatic lymph nodes had a sensitivity of 46%, specificity of 76%, positive predictive value of 41%, negative predictive value of 79%, and false-negative rate of 20%. Of the 97 patients whose ultrasound findings were indeterminate, lymph node metastasis was eventually detected in 33 patients (34%).
The results of the analysis of the yield of ultrasound-guided core-needle biopsy are shown in Table 2. Of the 114 patients whose findings were indeterminate or positive, 104 underwent core-needle biopsy. Of the 87 patients with an indeterminate result who underwent core-needle biopsy, the result was negative in 73 (83.9%) and positive in 14 (16.1%) (sensitivity=42%; specificity=84.4%). Of the 17 patients whose ultrasound results led us to suspect malignancy and in those who underwent UGB, the result was negative in 8 patients (40%) and positive in 9 (45%) (sensitivity=64%; specificity=66.7%). The biopsy material was insufficient in 3 of the patients with an indeterminate result and in 3 of the patients with a positive result (Fig. 3).
Overall, the sensitivity of core-needle biopsy was calculated to be 22.5%, specificity 19.8%, positive predictive value 100%, and negative predictive value 74.6%. Of the total number of patients who underwent core-needle biopsy with sufficient material for analysis, the percentage of positive nodes was 16.6% (14/84) in patients with an indeterminate ultrasound result and 64% (9/14) in those with a positive ultrasound result.
SLNB was performed in 361 patients: the result was negative in 282 cases and positive in 79. The percentage of positive sentinel nodes with a negative core-needle biopsy result (false negatives) was 22.8% (16/70) in the group with indeterminate ultrasound findings and 60% (3/5) in the group with positive ultrasound findings.
An evaluation of the 55 false negatives by ultrasound revealed a statistically significant percentage of false negatives in patients aged >60 years and for a Breslow thickness of <2mm. Findings for the remaining factors studied were not statistically significant (Table 4).
Results of the Cost-effectiveness Analysis.
| Protocol A SLNB | Protocol B UGB±SLNB | |
|---|---|---|
| Unit cost (€/patient) | 1700.00 | 1716.30 |
| Effectiveness | 0.06 | 0.21 |
| Cost-effectiveness | 28 605.00 | 8095.24 |
| Incremental cost | 16.30 | |
| Incremental effectiveness | −0.15 | |
| Incremental cost-effectiveness | −20.509.76 | |
| Dominated strategy | Dominant strategy | |
Abbreviations: SLNB, sentinel lymph node biopsy; UGB, ultrasound-guided biopsy.
Finally, we analyzed the costs generated by ultrasound, and the resulting UGB, before SLNB. Our results showed that SLNB was obviated in 23 patients (6%). The activity-based costing approach revealed a unit cost of €1700.00 per patient staged by SLNB alone and €1716.30 per patient staged by SLNB preceded by ultrasound. In terms of cost-effectiveness, the analysis revealed ultrasound followed by SLNB to be the dominant strategy, with a cost-effectiveness ratio lower than the alternative, ie, SLNB alone (€8095.24 vs €28 605.00 €) (Table 3).
DiscussionOur study confirms data reported in the literature with respect to the value of ultrasound as a method for locoregional staging of melanoma, namely, the method is useful, although not sufficiently accurate to replace SLNB as the technique of choice.7-17 It is noteworthy that lymph node metastasis was detected in 34% of patients with indeterminate ultrasound results. In these patients, the characteristics of the ultrasound examination neither confirmed metastasis nor enabled it to be ruled out. Furthermore, UGB made it possible to detect metastasis in more than half of all patients with positive ultrasound results. Given that UGB also presented a percentage of false negatives, a negative result indicates that staging should be completed using SLNB.
The main advantage of UGB before SLNB is that it obviates SLNB in cases where metastasis is detected using UGB alone. In our series, UGB obviated SLNB in 23 of 384 patients (6%). Even though this is not a large number, UGB before SLNB is considerably advantageous for patients who could benefit from it, since SLNB, despite being a minimally invasive procedure, is not free from complications.
Furthermore, the cost-effectiveness analysis showed that, for the cases in the present series, ultrasound for initial staging in patients with melanoma generates an additional cost of €16.30 per patient. This cost represents a 0.96% increase in the cost of staging patients with melanoma and is an additional cost that can be considered acceptable for most health services, especially if we take into account the low-to-medium incidence of melanoma in Spain (8.76; 95% CI, 7.50-10.02).23 Thus, in the case of a hospital with a reference population of 500 000 inhabitants and an expected incidence of 40-50 cases/year and assuming that 50% of cases are diagnosed as melanoma in situ or T1, with no indication for SLNB, the inclusion of ultrasound during initial staging would represent an annual total extra cost of between €350 and €450, which most health services can afford.23,24 In addition, the cost of performing ultrasound on all of the patients (n=384) and the lymph node biopsies performed to confirm ultrasound findings suspected of being malignant (n=104) was partially compensated by the 23 SLNBs avoided (€42 560 vs €39 100). This increase in total costs translated into an increase of €150.43 for each SLNB avoided, ie, 8.85% of the cost of performing SLNB. Despite the low morbidity of SLNB, the additional cost of €150 for obviating the procedure could be considered acceptable, thus favoring the implementation of ultrasound.
Similarly, taking the identification of lymph node metastasis as a measure of effectiveness, the extra unit cost of €16 was easily compensated by the high frequency of identification of lymph node metastasis in patients with a positive lymph node ultrasound result. This advantage of effectiveness (0.06 vs 0.21) generated an incremental cost-effectiveness ratio that showed the staging protocol based on ultrasound and SLNB to be the more cost-effective strategy and, therefore, better than staging by SLNB.
The limitations of the present study are that it was performed at a single center and, as a retrospective study, lacked randomization and a control group. With respect to core-needle biopsy, we obtained lower sensitivity and specificity than other authors, thus necessitating an analysis of the possible causes and a plan for improvement in causes that can be rectified. A potential explanation could be that the patients in the present study had a lower Breslow thickness than in other studies. They may have even had a lower metastatic burden, with greater difficulty for obtaining a suitable sample in core-needle biopsy; this hypothesis could be supported by the greater percentage of false negatives found in thinner tumors. Evaluating such a hypothesis would require a subgroup analysis and subsequent comparison with the results of other authors.
In conclusion, ultrasound—including UGB when necessary—is a useful and cost-effective diagnostic tool for staging melanoma before SLNB.
Ethical DisclosuresProtection of Humans and AnimalsThe authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of DataThe authors declare that they have followed their institutional protocols on publication of patient data.
Right to Privacy and Informed ConsentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
These authors have contributed equally to the article.
Please cite this article as: Olmedo D, et al. Valor de la ecografía ganglionar previa a la biopsia del ganglio centinela en 384 pacientes con melanoma: análisis de coste-efectividad. Actas Dermosifiliogr. 2017;108:931–938.