Although hidradenitis suppurativa is a common and serious skin condition, its treatment is not well established. It is now accepted that the moderate and severe forms of the disease are associated with marked systemic inflammation. The goal of treatment in hidradenitis suppurative is therefore to achieve systemic control of inflammation. In some cases, surgery may also be necessary to reduce the severity of the manifestations of cutaneous inflammation. Recent advances in our understanding of hidradenitis suppurativa have been accompanied by the emergence of novel approaches to its treatment, including the use of certain biologic drugs. Several clinical trials have been undertaken to test the effects of biologics (mainly adalimumab) in this setting. In this review, we analyze the different treatments available for hidradenitis suppurativa.
A pesar de la importancia y de la gravedad de la hidradenitis supurativa, el tratamiento de esta enfermedad no se encuentra bien definido. Hoy en día, la hidradenitis es considerada una enfermedad cutánea que principalmente en las formas moderadas y severas se asocia a un marcado componente inflamatorio sistémico. Por lo tanto, el tratamiento de esta enfermedad irá enfocado hacia un manejo sistémico del control de la inflamación, que ocasionalmente irá acompañado de la intervención quirúrgica para reducir la carga de inflamación localizada en la piel.
Los recientes avances en el conocimiento de la enfermedad se han acompañado de novedades terapéuticas, especialmente representadas por el desarrollo de ensayos clínicos de determinadas terapias biológicas, principalmente adalimumab, orientados al tratamiento específico de esta enfermedad.
En la presente revisión se pretende analizar las diferentes alternativas terapéuticas existentes en el manejo de la hidradenitis supurativa.
Hidradenitis suppurativa (HS) is currently considered an inflammatory disease of the pilosebaceous follicle with an underlying immune system imbalance that affects genetically predisposed individuals. The course of disease can be modified by exogenous triggers or aggravating factors.1,2
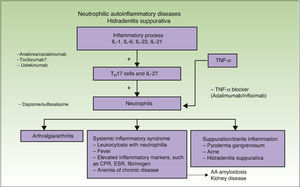
The association between HS and autoimmune and autoinflammatory diseases, such as pyoderma gangrenosum and Crohn disease (Fig. 1),1 together with clinical and laboratory findings, supports the existence of an immune system imbalance and consequently suggests inadequate control of the inflammatory response around hair follicles in intertriginous areas.
HS represents a true therapeutic challenge, with dermatologists responsible for taking decisions regarding patients’ treatment needs.
General MeasuresNumerous general measures can be taken to reduce situations that trigger flares, including tobacco cessation, weight reduction, control of cardiovascular risk factors, avoidance of the use of irritants in affected areas, and hair removal using lasers rather than razors.1 These measures should be complemented by adequate psychological support, which in some cases will need to be intensified.
Local TreatmentThe main noninvasive local treatment for localized Hurley stage I or mild stage II lesions is topical clindamycin 0.1% applied every 12hours.1–3 In one clinical trial, oral tetracycline administered at 500mg/12h did not show superior results to topical treatment with clindamycin.4 Topical resorcinol 15% has also proven to be effective in reducing the pain and duration of inflammatory lesions in patients with Hurley stage I or II lesions.1–3 Intralesional corticosteroids are the most common invasive local treatment and the most widely used drug is slow-release triamcinolone acetonide (depot preparation 40mg/mL). Triamcinolone acetonide injections result in the remission of inflammatory nodules within 48 to 72hours in patients with acute local lesions.1
First-Line Systemic/Biologic TherapiesFirst-line treatments for HS are treatments supported by high levels of evidence and favorable results.
Systemic TreatmentsCombined Treatment With Oral Clindamycin and Oral RifampicinThe combined use of clindamycin 300mg/12h and rifampicin 300mg/12h for 10 weeks is one of the most common treatments used to induce remission in patients with HS, regardless of disease severity (Hurley stage I, II, or III).1 The beneficial effects of this combination of antibiotics have been confirmed by all series published to date, including a report of 116 patients.5,6 The therapeutic effect is attributable to the anti-inflammatory properties of the 2 drugs and probably also to their ability to destroy the biofilm mentioned in the first part of this review article. The combination is well tolerated, as the most common adverse effects are gastrointestinal discomfort and diarrhea (generally mild).1
Other antibiotics used to treat HS are doxycycline, minocycline, and rifampicin associated with moxifloxacin and/or metronidazole, with variable response.1–3
Oral AcitretinThe use of acitretin in the treatment of HS is justified by the involvement of psoriasiform hyperplasia in the etiology and pathogenesis of the disease. In a recent study (2014), Matusiak et al.7 reported on the efficacy of acitretin (mean [SD] dose, 0.56 [0.08] mg/kg/d) in 17 patients with HS. Nine of the patients completed the 9 months of treatment, and 8 (47%) achieved a reduction of 50% from baseline in the Hidradenitis Suppurativa Severity Index. The authors concluded that acitretin appears to be a promising option for the management of HS, although they cautioned that its use might be limited by the high doses required.
Isotretinoin has not proven to be effective in HS, probably because it primarily causes atrophy of hypertrophic sebaceous glands, which are seen in juvenile acne, but not in HS.1,7
DapsoneDapsone is a nonteratogenic sulfone antibiotic that has antibacterial and anti-inflammatory (mainly antineutrophilic) properties. In a study of 24 patients with Hurley stage I and II disease, treatment with dapsone at doses of between 50 and 200mg/d resulted in significant clinical improvement in 38% of patients.8,9
Biological TherapiesAccording to the available evidence, the most effective tumor necrosis factor α (TNF-α) blockers in HS are adalimumab and infliximab.10
AdalimumabAdalimumab is supported by the highest levels of evidence (including data from randomized clinical trials) and is considered the most specific treatment for HS.10–12 Based on the data available, it is currently the main drug for the treatment of refractory Hurley stage II disease or moderate to severe Hurley stage III disease.
Dermatologists are familiar with the use of biologic therapy in the setting of cutaneous and articular psoriasis. The standard doses of adalimumab in these cases are 80mg at week 0, 40mg at week 1, and 40mg every other week thereafter. There are, however, clear differences between psoriasis and HS, mainly related to the level of inflammatory activity (Table 1). In the setting of TNF-α-dependent inflammatory disorders, gastroenterologists use adalimumab administered at doses that are twice as high as those used in psoriasis to treat inflammatory bowel disease (IBD), and Crohn disease in particular, as this has a higher inflammatory burden. The standard regimen used in this setting is 160mg at week 0 and 80mg at week 2, followed by 40mg administered every week or every 2 weeks, as appropriate. According to the latest data available, HS appears to be closer to the inflammatory spectrum of IBD than of psoriasis, and therefore requires treatment with higher doses of adalimumab than those typically used in dermatology; the response rates would be expected to be similar to those seen in IBD.
Differences Between Cutaneous Psoriasis and Hidradenitis Suppurativa That Influence Treatment of the Latter.
| Cutaneous psoriasis | Hidradenitis suppurativa |
|---|---|
| Less inflammation and easier to control | Greater inflammation and more difficult to control |
| Does not hurt but can cause itching | Painful and oozes pus (bad smell and stains clothes) |
| Not disabling (except for psoriatic arthritis) and does not cause scarring | Disabling and leaves permanent scars |
| Less impact on quality of life | Greater impact on quality of life |
| Cannot degenerate into cancer | Cancer can arise (chronic inflammation) in HS lesions, predominantly in the perianal and gluteal region |
| Classic and biologic therapies (with summary of product characteristics and supported by studies with higher levels of evidence) | Adalimumab, only treatment with recommended approval from the European Medicines Agency following completion of randomized clinical trials |
Results from the latest trials of adalimumab and HS have shown that, just as in IBD, higher induction and maintenance doses than those used in psoriasis are needed to achieve better disease control; the proposed regimen is 160mg at week 0, 80mg at week 2, and 40mg a week from week 4 onwards.12–15 These higher doses are justified by the higher levels of TNF-α found in lesional skin in HS compared with psoriasis (Fig. 2).16
Reports on treatment efficacy for HS are also more similar to those published for IBD than for psoriasis. A retrospective Spanish study of the use of biologic therapy in HS reported complete, lasting remission in approximately 15% of patients and partial remission in approximately 50%.14 These data, however, should be interpreted with caution as most patients were administered lower doses than those recommended for HS and IBD.
In a double-blind, randomized, placebo-controlled trial of 154 patients with moderate to severe HS who did not respond to or tolerate tetracycline antibiotics, clinical response was achieved by week 16 in 17.6% of patients treated with adalimumab 40mg weekly, 9.6% of patients treated with adalimumab 40mg every 2 weeks (psoriasis regimen), and 3.9% of patients treated with placebo.12
In a more recent phase 3 randomized, placebo-controlled trial (PIONEER II) involving 326 patients, patients were treated with an induction dose of 160mg (week 0), followed by 80mg at week 2, and 40mg weekly starting at week 4. Response was evaluated using the Hidradenitis Suppurativa Clinical Response (HiSCR) measure, which is defined as a reduction of 50% or more in the count of inflammatory lesions (abscesses and inflammatory nodules) and no increase in abscesses or draining fistulas. At week 12, adalimumab proved to be significantly superior to placebo for the primary endpoint (HiSCR), with a satisfactory response observed in 50% of patients treated with this biologic. Efficacy was observed at week 2, and the adverse effects were similar to those seen in the placebo group and consistent with the safety profile of adalimumab.
InfliximabInfliximab is the biologic with the longest tradition in the treatment of HS and its use is supported by high levels of evidence, second only to adalimumab.17 As occurs with adalimumab, the best results are achieved with an intensified regimen of 5mg/kg per month17 (5mg/kg at weeks 0, 2, and 6, and monthly thereafter). The main disadvantage of infliximab compared with adalimumab is the need to use an intensified regimen, as a majority of patients with HS are overweight or obese. Furthermore, infliximab infusions need to be administered at a day hospital. Formation of antidrug antibodies may be responsible for the reduction in efficacy observed in certain patients with HS treated with infliximab, but levels have not yet been analyzed in this setting.
Second-Line Systemic/Biologic TherapiesTreatments supported by a lower level of evidence or associated with less favorable results are considered second-line treatments for HS.
Systemic TherapyHormone TherapyThere have been isolated case reports of HS responding to contraceptive drugs, such as cyproterone, and antiandrogens, such as finasteride.6,18 Randhawa et al.19 published a report on 3 pediatric patients with HS who responded favorably to finasteride.
In a report on possible medical treatments in 350 patients with HS, Scheinfeld18 considered that it would be interesting to investigate the use of dutasteride 0.5mg/d, as it is a more potent blocker of 5α reductase isoenzyme than finasteride, and in addition, it blocks the type 1 isoenzyme, unlike finasteride.6,18 There are, however, no publications on its use in HS.
Systemic CorticosteroidsPatients with HS, like patients with other inflammatory diseases, experience clinical improvement with systemic corticosteroids. Treatment, however, is limited to short cycles due to the risk of long-term adverse effects. There are no standardized regimens for the use of systemic corticosteroids in HS.6,18
CiclosporinCiclosporin, a calcineurin inhibitor, is a potent immunosuppressant that is very active in inflammatory skin diseases. Its targets include T lymphocytes, interleukin (IL) 2, and TNF-α. Contrasting with the situation in psoriasis, only a few isolated reports showing the efficacy of ciclosporin in severe HS have been published.1 The potential of this immunosuppressant in HS should be studied in clinical trial settings.
MethotrexateMethotrexate has been described as an ineffective drug when used in isolation in HS,20 but it has not been widely studied. It is frequently used in association with TNF-α blockers, and this combination is therefore supported by robust safety data. It would be interesting to evaluate its synergic effect with adalimumab and its potential for lengthening the therapeutic effect of this drug, as it does in psoriasis.
AlitretinoinAlitretinoin is a retinoid whose therapeutic potential in HS should be investigated for numerous reasons: it has lower teratogenic potential than acitretin due to its shorter half-life and is therefore suitable for use in women of childbearing age. It also has greater immunomodulatory effects due to its rexinoid effect. While it is not cheap, it is not more expensive than biologics, and finally, it is not an immunosuppressant.
In an Italian study of 14 patients with HS, alitretinoin administered at 10mg/d for 24 weeks resulted in clinical improvement in 78.5% of patients.21 It would also be interesting to investigate response rates for the 30-mg/d dose.
Other TreatmentsAlternative treatments supported by lower levels of evidence for HS include metformin 500mg/8h,1 sulfasalazine 1g/12h,1 and tacrolimus.22
Biologic TherapiesAnakinra/CanakinumabAnakinra is an IL-1 receptor antagonist. Although it is indicated for use in rheumatoid arthritis, it is now largely used as an orphan drug for the treatment of autoinflammatory diseases. IL-1 is a proinflammatory cytokine that is very closely associated with sterile inflammation and neutrophils,1,23 and hence neutropenia features among the drug's possible adverse effects. Response to anakinra has been reported in isolated cases of HS and in an open-label study of 6 patients.1 However, there have also been reports of treatment failures,1 possibly due to the high levels of IL-1 detected in HS lesions, i.e., the drug may lack the ability to elicit a response in certain patients (Fig. 2). Anakinra is normally administered subcutaneously at a dose of 100mg/d, but there has been a report of a patient responding to a dose of 200mg/d.1 Local reactions are the main problem associated with the use of anakinra, although these tend to improve after 4 weeks of treatment. Anakinra should not be used in association with TNF-α blockers.
Canakinumab is a fully human IgGκ monoclonal antibody targeting IL-1β. It selectively binds to IL-1β with high affinity, neutralizing its biological activity by blocking interaction with IL-1 receptors and preventing the production of inflammatory mediators. It is indicated in autoinflammatory syndromes, systemic juvenile idiopathic arthritis, and arthritic gout. It has an advantage over anakinra is that it is administered subcutaneously every 4 or 8 weeks. There has been a report of satisfactory response to canakinumab in a patient with HS and pyoderma gangrenosum.23 However, its high cost is a deterrent for the conduct of larger studies in HS.
UstekinumabUstekinumab is a biologic that targets the p40 subunit of IL-12/IL-23. It has proven effective in the treatment of isolated cases of HS.1 Like adalimumab and infliximab, it is probably more effective when administered at an intensified regimen consisting of 90-mg doses every 2 months; this is supported by a report of treatment failure when administered at the lower dose used for psoriasis.25
Surgical TreatmentSurgery is indicated for the treatment of nodules and isolated fistulas and for severe, extensive disease that does not respond to medical treatment.1,26,27 Its effectiveness, however, has not yet been evaluated in clinical trials.27 Additionally, while the literature shows that surgery appears to achieve good results in milder forms of HS, high recurrence rates have been observed in patients with moderate or severe disease and high levels of cutaneous and systemic inflammation treated with surgery only.
Several surgical techniques are indicated for HS:
- 1.
Incision and drainage
- 2.
Unroofing and marsupialization
- 3.
Localized excision
- 4.
Wide excision
The choice of surgery and size of margins are determined by the area affected and the degree of involvement.
Preoperative care. Prior reduction of inflammation is recommended in patients with high degrees of inflammation and unclear margins. A 10-to-12-week course of antibiotics is probably sufficient for mild and moderate cases, but a short cycle of oral corticosteroids can also be added. Patients with severe disease can be administered prednisone 40-60mg/d for 2 to 3 days followed by a decreasing dose over an additional 10 to 12 days. Ciclosporin has also been used, as have TNF-α blockers. General hygiene and dietary measures are recommended in all cases.
Incision and DrainageIncision with drainage is a simple technique that can be performed under local anesthesia on an outpatient basis. It tends to result in rapid relief of pain in the case of isolated nodules, but recurrence is common.28,29
Punch debridement has also been proposed as a modification of this technique.30 This procedure involves centering a biopsy punch with a diameter of 5 to 7mm over an inflamed pilosebaceous unit, which is then debrided by digital pressure followed by curettage. The goal is to remove the remains of the sebaceous gland and/or the follicle containing cells involved in the generation of fistulas and fibrous tracts. Preliminary data suggest that recurrences are relatively infrequent with this technique.
UnroofingUnroofing (or deroofing) with marsupialization is a simple technique that can also be performed on an outpatient basis.31 In this technique, the fistulous tract or roof of a nodule is transfixed using a probe or a mosquito forceps; the tissue is then removed with the aid of a scissors, electric scalpel, or radiofrequency ablation, thereby exposing the bed of the lesion, which is scraped with a curette. The lesions are left to heal by secondary intention. Unroofing is suitable for recurrent painful stage I or II lesions, and results in acceptable cosmetic results. Approximately 17% of lesions treated using this technique have been found to recur within a mean of 4 to 6 months.31,32
Local ExcisionLocal excision has the same advantages and disadvantages as incision and drainage.
Wide ExcisionWide excision involves removing an entire affected area with margins extending beyond the visibly affected region. When used in combination with medical measures and treatments, wide excision is the technique that is most likely to achieve disease control in patients with chronic, extensive stage III disease.1–29 The surgical defect can be reconstructed using simple local or free flaps, skin grafts, tissue expanders, or simply closure by secondary intention.33 Assuming that adequate margins can be guaranteed, the reconstruction method does not influence recurrence rates and should therefore be chosen according to the site of excision and the size of the lesion.34,35 Margins of between 0.5cm (axillae) and 1.5cm are recommended. Deep excision extending as far as the fascia or at least 5mm of fat is important to ensure removal of the deep coils of the apocrine glands. Wide excision, however, does not protect against recurrence at distant apocrine sites.
Several authors advise against using primary closure techniques due to the high risk of recurrence (54%-69.9% vs 13% for grafts and 18% for local flaps), but these higher rates are attributable to the higher number of affected margins or to incomplete resection.26 Nonetheless, significant differences have been reported for recurrence rates associated with different reconstruction techniques, but it is difficult to compare modalities due to the nature of HS and the number of techniques that exist. Some studies that have assessed recurrence rates by location have reported lower rates in the axillae (3%) and perianal area (0%) than in the groin and perineal region (37%) and submammary area (50%), suggesting that recurrence is more common at sites with larger areas of apocrine glands.26
Vacuum-assisted closure involves the use of a device that delivers negative pressure to the wound and therefore promotes blood flow, increases the rate of granulation tissue formation, and facilitates wound drainage, thereby reducing bacterial load. This technique has been found to produce better results and lower rates of recurrence when used in larger wounds.36
Lasers and LightA range of laser and light systems have been used in HS, with varying results. Carbon dioxide laser therapy results in improvement by clinically vaporizing all the layers of tissue as far as the deep subcutaneous fat or fascia. Recurrence is low.37 Hair removal lasers and intense-pulsed light systems also result in clinical improvement by reducing the number of hair follicles and associated inflammation.38 Long-pulsed (1064nm) Nd:YAG 1064 laser therapy has also been described as effective for the treatment of Hurley stage II and III disease.39 Finally, reports describing the use of photodynamic therapy in series of patients with HS have increased,40–42 but the results are varied and recurrence is high. A standardized photodynamic treatment regimen has not been described.
Other TreatmentsRadiation therapy is no longer used to treat HS due to the risk of tumors and the existence of alternative treatments.43 However, classic studies of this technique show complete remission in 38% of cases and improvement in 40%. Cryotherapy has proven effective in the management of isolated cases of painful HS nodules, as has cryoinsufflation, which involves applying liquid nitrogen into small fistulous tracts using a needle that follows the path of the fistula, followed by 2 freeze cycles in the entire area.43,44 Unfortunately, recovery time is long and the treatment is quite painful.
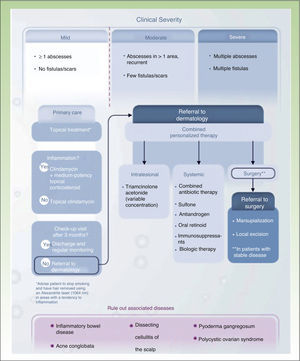
Conclusions: Towards a Treatment AlgorithmOur review of the different treatment options for HS shows that a) HS is a chronic skin disease with a significant systemic component (largely in moderate and severe forms of the disease) that needs to be controlled medically; b) HS is an inflammatory disease that needs to be treated with a combination of topical and systemic/biologic treatments and, occasionally, surgery of varying levels of complexity; and c) HS is a disabling disease whose management can be optimized by designing personalized therapies overseen by dermatologists with the involvement of primary care physicians, surgeons, and nursing staff, and occasional collaboration from other specialists, such as psychologists and gastroenterologists, among others (Fig. 3).45 Recent data regarding the efficacy of adalimumab in HS, combined with the recent approval of this drug by the European Medicines Agency as a primary specific treatment for HS, will help us to manage cases that require maintenance therapy to achieve optimal control of inflammation.
Treatment algorithm for hidradenitis suppurativa.
Source: Martorell.45
The authors declare that they have no conflicts of interest.
Please cite this article as: Martorell A, García FJ, Jiménez-Gallo D, Pascual JC, Pereyra-Rodríguez J, Salgado L, et al. Actualización en hidradenitis supurativa (ii): aspectos terapéuticos. Actas Dermosifiliogr. 2015;106:716–724.