Frontal fibrosing alopecia (FFA) is an increasingly common acquired primary scarring alopecia, first described by Kossard in 1994. Clinically it is characterized by frontotemporal hairline recession, frequently accompanied by eyebrow loss. FFA was initially thought to have a hormonal origin as it was first described in postmenopausal women and premenopausal women with a history of hysterectomy or early menopause. This origin, however, has been questioned in recent years due to the publication of cases in men and premenopausal women. Although FFA has a highly characteristic clincal pattern, it is histologically similar to lichen planopilaris, and is currently believed to be a clinical variant of this condition. No clinical trials to date have investigated the efficacy of treatments for FFA. Numerous drugs, however, have been assessed in observational studies, and the best results to date have been reported for 5-αreductase inhibitors and intralesional corticosteroids, followed by antimalarials and calcineurin inhibitors. In this article, we review the latest data on the etiology, pathogenesis, clinical presentation, diagnosis, and treatment of FFA.
La alopecia frontal fibrosante (AFF) es un tipo de alopecia cicatricial primaria adquirida, descrita por Kossard en 1994, cuya incidencia ha aumentado en los últimos años. Se caracteriza clínicamente por una recesión de la línea de implantación frontotemporal del cabello, acompañada frecuentemente por alopecia de las cejas. La AFF fue inicialmente descrita en mujeres posmenopáusicas y premenopáusicas con antecedentes personales de histerectomía o menopausia precoz, por lo que se propuso un origen hormonal de la enfermedad. Sin embargo, en los últimos años se han publicado estudios en varones, así como en mujeres premenopáusicas que cuestionan dicha etiología. A pesar de que las manifestaciones clínicas de la AFF son muy características, desde el punto de vista histopatológico los hallazgos son similares al liquen plano pilaris, por lo que actualmente es considerada como una variante clínica de este último. Hasta el momento no se han realizado ensayos clínicos sobre las diferentes alternativas de tratamiento en pacientes con AFF. En los estudios observacionales publicados se valora el uso de múltiples fármacos, siendo los inhibidores de la 5-alfa-reductasa y los corticoides intralesionales los que mejor resultado han obtenido hasta el momento, seguidos por los antipalúdicos y los inhibidores de la calcineurina. Esta revisión analiza de forma exhaustiva la información más recientemente publicada sobre la etiopatogenia, la clínica, el diagnóstico y el tratamiento de los pacientes con AFF.
Frontal fibrosing alopecia (FFA) is an increasingly common acquired primary cicatricial alopecia,1 first described by Kossard in 1994.2 Clinically, it is characterized by frontotemporal hairline recession, frequently accompanied by eyebrow loss.2,3 As this is a cicatricial alopecia, hair loss is irreversible, with a major impact on the confidence and quality of life of the affected patients.4 Although the clinical manifestations of FFA are very characteristic, the histopathological findings are identical to those observed with lichen planopilaris (LPP),3 and so some authors consider FFA as a variation of LPP.1
PathogenesisFFA mainly affects postmenopausal women with a mean age of 60 years, although it has also been reported in men5–10 and premenopausal women.8–14 Indeed, the youngest woman reported to have the condition was 23 years old.10 Despite the notable increase in incidence since it was first described,2 the etiology of FFA is still unknown. The role of sex hormones in the development of FFA has been proposed by several authors. These chemicals are thought to act by inducing a decrease in hair growth resulting from stimulation of the transition of hair follicles from anagen to telogen.15,16 Subsequent studies have supported this hypothesis in that a favorable response has been obtained with the use of antiandrogen drugs in patients with this disease.10,11,16–18 Furthermore, among patients with FFA, there is a high proportion of patients with early menopause (14% versus 6% in the general population),10 hysterectomy patients (11%-21%),10,12,14 associated androgenetic alopecia (AGA) (40% in some series),10,15 and oophorectomy (although no consistent association has been demonstrated to date).19 However, studies published in men and in premenopausal women have brought into question this hormone theory. Of note, the pathogenic mechanism for AGA and FFA differs (miniaturization versus inflammation and fibrosis).20 It has also been demonstrated that hormone replacement therapy does not prevent or slow disease progression3 and hormone studies in patients with FFA have confirmed that androgens are not elevated and other hormone imbalances are not present in peripheral blood.3,19
The association of FFA with autoimmune diseases such as hypothyroidism (11%-23% of patients with FFA versus 4.2% in the general population),9,10,12,13 vitiligo,21 or Sjörgren syndrome13 may point an autoimmune origin. In the case of LPP, good response to corticosteroid therapy, presence of antibodies in some cases, and the influence of certain drugs in the presentation of epitopes in the hair follicles to the immune system have been reported. However, FFA differs from LPP in this point, as no specific antibodies have been detected with sufficient titer in these patients, and to date, there is no evidence in the literature to support the autoimmune origin of the disease.22
Likewise, it has not been possible to demonstrate a genetic component in the pathogenesis of FFA, although a family association has been suggested by some series, with a reported family history in approximately 8% of the patients.9,10,21,23 A recent study of 4 families affected by the disease with 8 cases in mother and daughter with FFA found that all mothers had postmenopausal FFA and all daughters had developed the disease before menopause, suggesting that family history could be associated with an earlier presentation of the disease.24
Finally, although reports have been published of isolated cases of FFA associated with surgical procedures such as hair transplantation or face lifts, in which an immune response secondary to a Koebner process was postulated as a possible etiologic factor, the number of patients is still too small to establish a consistent relationship.3,14,25,26
The pathogenesis of FFA, like its etiology, is still unclear. There would appear to be a set of multiple processes that trigger and lead to disease progression. Laboratory experiments in mice have shown that FFA reversibility depends exclusively on damage to epithelial stem cells in the hair follicles.27 Notable, the highest density of inflammatory infiltrate is observed around these cells. In addition, decreased expression of cytokeratin 15, a marker of epithelial stem cells in hair follicles, has been observed in FFA biopsies.28,29 Epithelial stem cells are located in the region of the hair follicle known as the bulge. This region constitutes a niche with immune privilege,4,22,28,30,31 as it is surrounded by T lymphocytes, Langerhans cells, macrophages, and antimicrobial peptides such as β-2-defensin, psoriasin, cathelicidin, and RNAase 7, which block the passage of toxic external agents.32 The above processes, along with local immunosuppression induced by the absence of major histocompatibility complex (MHC) class i and ii molecules and β-2-microglobulin in stem cells4,33 and by secretion of endogenous immunosuppressants such as transforming growth factor β, melanocyte-stimulating hormone α, and cortisol,31,33,34 impede excessive inflammation that may damage stem cells in the hair follicles. Along these lines, Harries et al.35 demonstrated an increase in MHC class i and ii molecules, β-2-microglobulin, and interferon γ in areas of alopecia in patients with FFA.4 Stem cells would thus be exposed to cytotoxic T cells of the inflammatory infiltrate, leading to their destruction.
The role of sebaceous glands in the pathogenesis of FFA has also been investigated by different authors, as a notably higher rate of loss of these glands is observed in cicatricial alopecias than in noncicatricial alopecias (55% and 5%, respectively).36 In normal conditions, sebaceous glands have an impact on changes undergone by the outer root sheath during the hair cycle,37,38 such that disturbance may favor the development of cicatricial alopecia. In patients with FFA, inflammation of the gland ducts due to cytotoxic damage has been observed and this might contribute to the development of the disease.36 In addition, recent studies have demonstrated that mice with deficiency in peroxisome-proliferator-activated gamma (PPAR-γ), a molecule that impacts lipid metabolism, have similar clinical manifestations to LPP, and so PPAR-γ is postulated as an important mediator in FFA and could be a molecular target for future therapeutic strategies.22,28,39
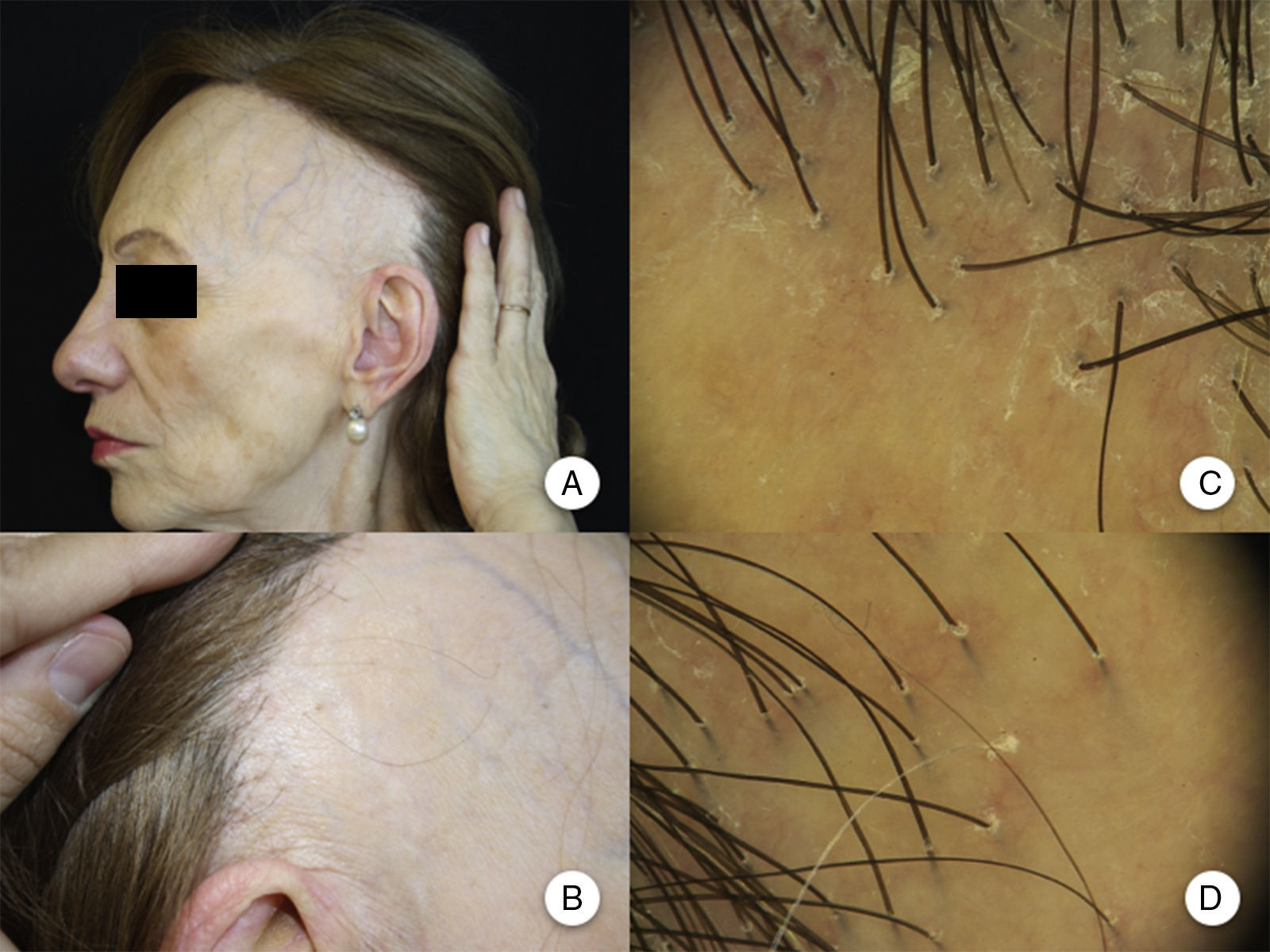
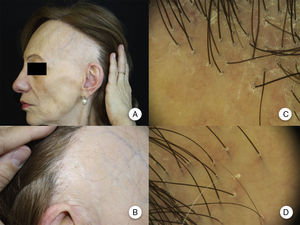
Clinical ManifestationsReceding HairlineThe most constant clinical manifestation is symmetric, bilateral, frontotemporal hairline recession.20 The recession may at times extend as far as the retroauricular region,3 although continuity between this region and the frontal band of alopecia does not need to be present (Figure 1A andB).13 The skin in the region of alopecia has irreversible scarring on the surface. Of note is the smoothness of the skin, with loss of follicular orifices and uniform paleness, in contrast to surrounding areas, which show pigmentation characteristic of chronic actinic damage in accordance with the age of the patient.2 In the area of transition between the 2 areas, interfollicular or perifollicular erythema can be observed in 50% of patients, with follicular hyperkeratosis in 30% to 60%, although these changes are not accompanied by induration or sclerosis.10,20
Clinical manifestations in frontal fibrosing alopecia. A, Receding hairline in the frontotemporal region, extending to the retroauricular region. B, The skin in the region of alopecia shows surface scarring, with loss of follicular orifices, uniform paleness, and a vascular network. C, Trichoscopy image at the frontal hairline, in which absence of follicular ostia, erythema, perifollicular scaling, and branched capillaries can be observed. D, Trichoscopy image of the retroauricular region in which similar changes to those described above can be observed.
The presentation of alopecia in FFA is usually asymptomatic, thus making early diagnosis more difficult.20 However, some patients may report pruritus and even pain, which is present in 20% and 35% of cases, according to the series studied.10 Moreover, although frontotemporal hairline recession is the most typical, studies have reported involvement of the occipital region in 7% and 14% of patients.10,13 In particular, the study by Vañó-Galvan et al.10 is the largest study to date, and the findings reflect a greater percentage of occipital involvement in women (33% versus 13%), although this difference is not statistically significant. These are important findings in terms of treatment, especially in the case of hair transplantation, which may be less effective if there is occipital involvement.40
The distance of hairline recession can range from 0.5 to 8cm,3 although approximately 75% of patients have recession of less than 3cm.10 In fact, Vañó-Galván et al.10 found a statistically significant correlation between the recession distance and the duration of the disease. Finally, the course of FFA is varied. Stabilization may occur without the need for treatment,3,11,12,17 or after withdrawal of treatment,14 or the disease may progress rapidly leading to clown-pattern alopecia,20 with involvement of the entire scalp.12
Eyebrow InvolvementInvolvement of the eyebrows is currently considered another clinical manifestation of FFA. Although not always present, eyebrow loss is observed in a high percentage of patients, with some studies finding rates of up to 95%.14 This manifestation is present as a clinical characteristic at onset in 20% to 48% of patients with FFA and preceded frontal alopecia by up to 8 years in one of the cases published.13 Therefore, eyebrow loss can assist in early diagnosis of the disease in 15% to 39% of patients with FFA, and it is usually associated with moderate forms of the disease.10,13
The degree of eyebrow loss is also variable, with the most characteristic presentation being hair loss in the lateral region, which may progress to total or almost total alopecia of both eyebrows. In cases in which loss is not complete, decreased eyebrow density is observed throughout the rest of the eyebrow, as is usually observed with alopecia areata, and this may hinder differential diagnosis of the 2 processes.20 Finally, although mild perifollicular erythema has been observed in some isolated cases, it is more common to observe eyebrow loss without significant clinical inflammation or evident scaling.13
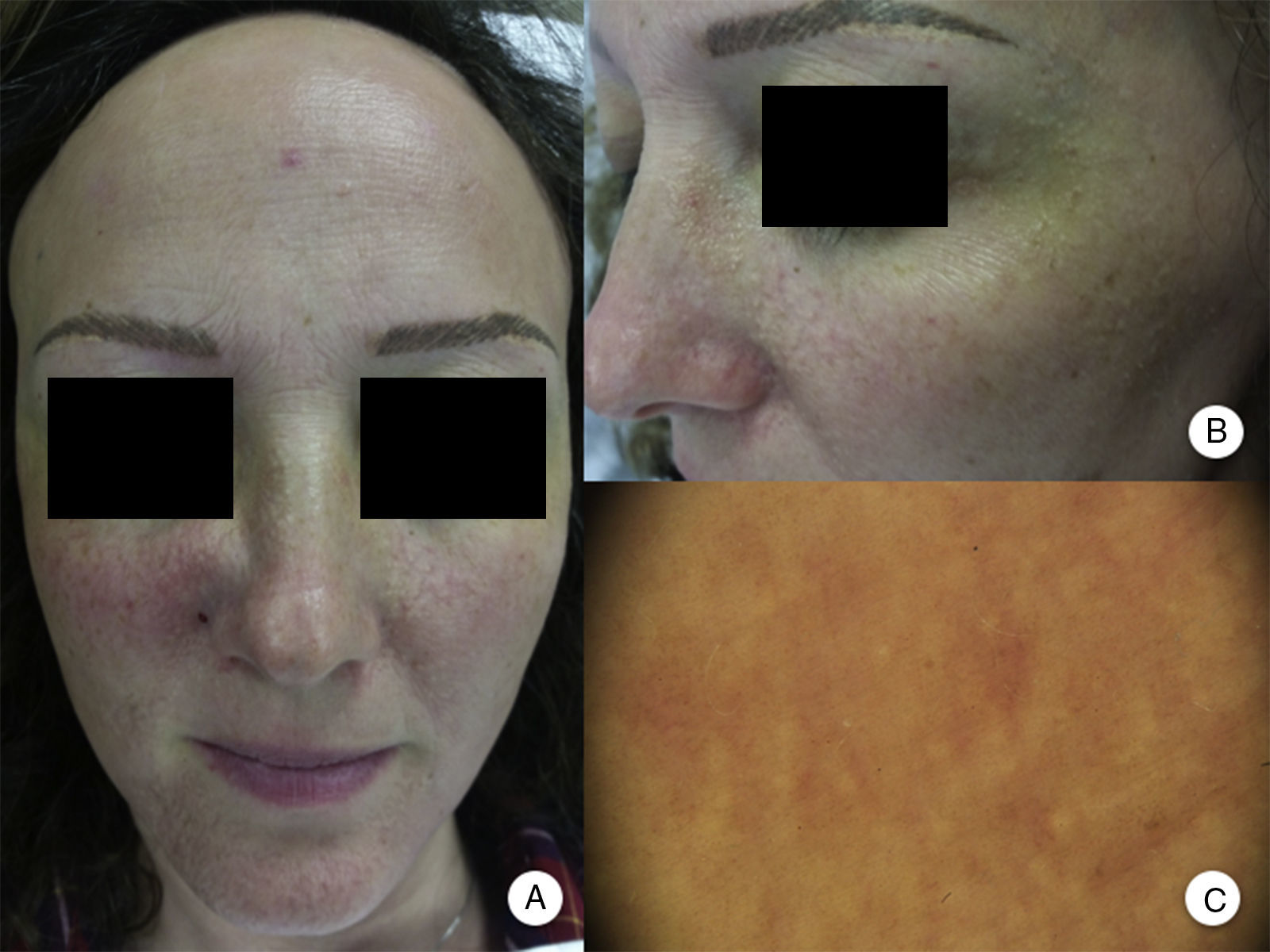
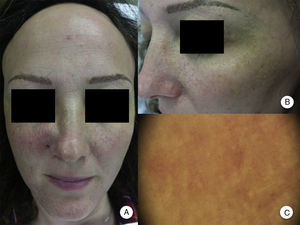
Involvement of Other Hairy AreasEyelash loss is observed in 14% to 26% of patients.10,14 Involvement of facial hair, as well as the presence of facial papules, has been reported in 6% to 37% of cases, while loss of facial hair occurs in 50% of men.10,12,14 Loss of body hair is observed in 25% of patients with FFA, with the most frequently affected areas being the axillas, the pubis, and the limbs.13 Furthermore, joint loss of body hair and eyelashes, along with the appearance of facial papules, has been associated with more severe forms of FFA, and some authors have considered this to be a prognostic indicator and the point at which systemic treatment should be initiated.10 In fact, although perifollicular papules have been reported frequently at the line of regression of alopecia of the scalp, facial papules in FFA are much less frequent and were not described until 2009 as monomorphic, noninflammatory, skin-colored papules with a corrugated and rough appearance, without erythema or associated scaling (Figure 2). These papules represent involvement of facial hair, contrasting with alopecia of body hair in FFA, which is subclinical.
Facial papules in frontal fibrosing alopecia. A, Noninflammatory, monomorphic, skin-colored follicular papules can be observed with a corrugated or rough appearance and without associated erythema or scaling. B, Detail of the facial papules. C, With dermoscopy, follicular white dots can be seen superimposed on the vascular network that is usually seen in the face.
As is the case with eyebrows, in body regions affected by FFA, inflammatory reaction, and scaling are not observed. Moreover, at times, complete alopecia does not occur. Instead, the process is manifest as decreased hair density in the region, associated with mild skin atrophy and perifollicular erythemia.2,11 Finally, the involvement of body hair may be accompanied by follicular hyperkeratosis at times, and so many patients finally diagnosed with FFA have been assessed in dermatology clinics for Graham-Little syndrome.
Other ManifestationsThe prevalence of AGA in patients with FFA has been analyzed in several studies under the hypothesis that both types of alopecia share a common hormonal origin; however, there is controversy in the literature on this point, as some studies do not find any relationship between FFA and AGA,11,13,14 whereas others report an association of up to 40% in patients overall and in 67% of male patients.10,17
Finally, other recently described clinical manifestations of FFA are glabellar red dots,41 depression of frontal veins,42 and pigmented facial macules.43
DiagnosisThe clinical manifestations of FFA once established are very characteristic; however, differential diagnosis with other types of alopecia may be complex when FFA is in its initial stages. In these cases, other complementary diagnostic techniques such as trichoscopy or histopathology may be useful.10,44Table 1 summarizes the main clinical and histopathological markers of FFA that can help in differential diagnosis with other alopecias.
Differential Diagnoses of Frontal Fibrosing Alopecia.
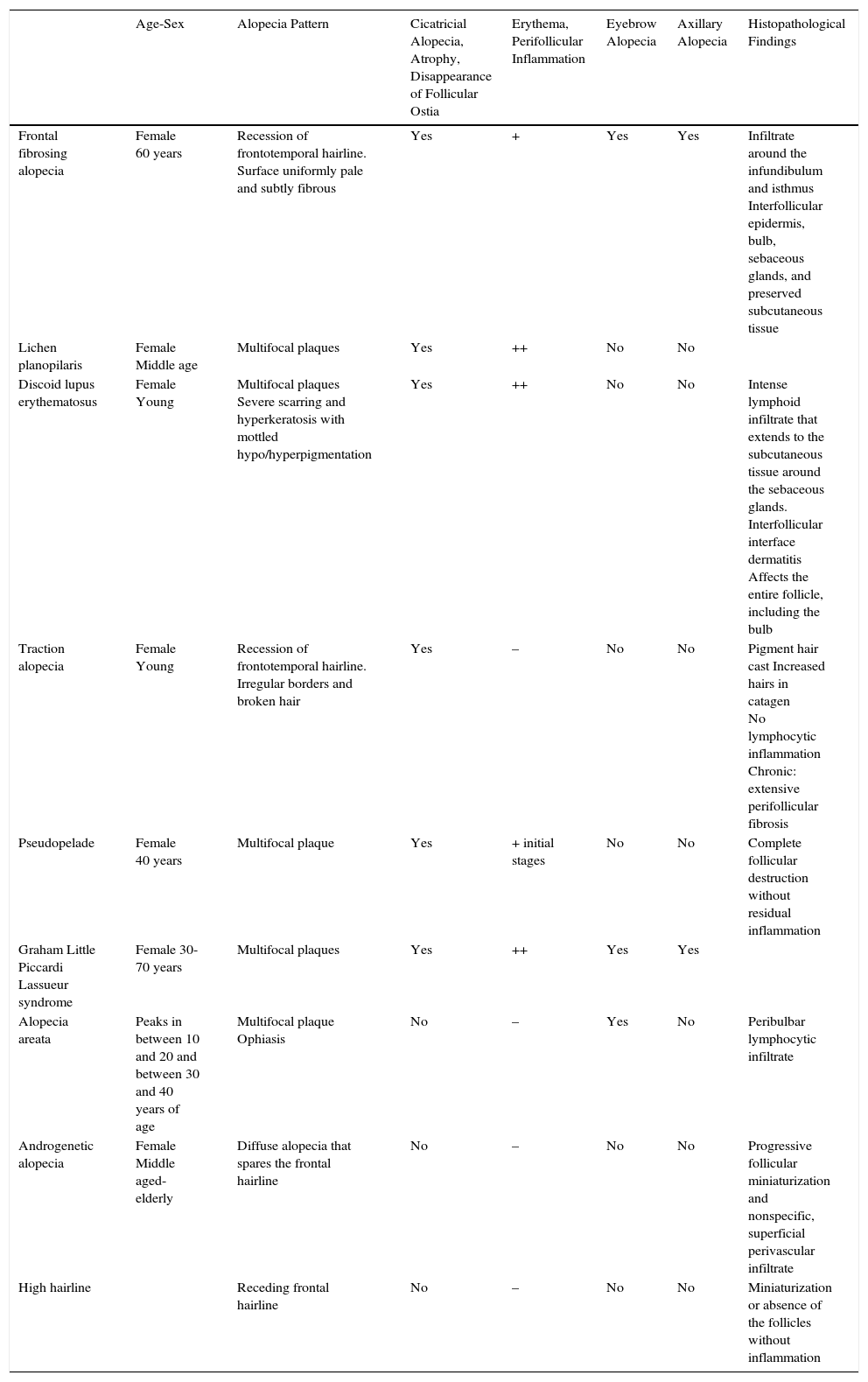
| Age-Sex | Alopecia Pattern | Cicatricial Alopecia, Atrophy, Disappearance of Follicular Ostia | Erythema, Perifollicular Inflammation | Eyebrow Alopecia | Axillary Alopecia | Histopathological Findings | |
|---|---|---|---|---|---|---|---|
| Frontal fibrosing alopecia | Female 60 years | Recession of frontotemporal hairline. Surface uniformly pale and subtly fibrous | Yes | + | Yes | Yes | Infiltrate around the infundibulum and isthmus Interfollicular epidermis, bulb, sebaceous glands, and preserved subcutaneous tissue |
| Lichen planopilaris | Female Middle age | Multifocal plaques | Yes | ++ | No | No | |
| Discoid lupus erythematosus | Female Young | Multifocal plaques Severe scarring and hyperkeratosis with mottled hypo/hyperpigmentation | Yes | ++ | No | No | Intense lymphoid infiltrate that extends to the subcutaneous tissue around the sebaceous glands. Interfollicular interface dermatitis Affects the entire follicle, including the bulb |
| Traction alopecia | Female Young | Recession of frontotemporal hairline. Irregular borders and broken hair | Yes | – | No | No | Pigment hair cast Increased hairs in catagen No lymphocytic inflammation Chronic: extensive perifollicular fibrosis |
| Pseudopelade | Female 40 years | Multifocal plaque | Yes | + initial stages | No | No | Complete follicular destruction without residual inflammation |
| Graham Little Piccardi Lassueur syndrome | Female 30-70 years | Multifocal plaques | Yes | ++ | Yes | Yes | |
| Alopecia areata | Peaks in between 10 and 20 and between 30 and 40 years of age | Multifocal plaque Ophiasis | No | – | Yes | No | Peribulbar lymphocytic infiltrate |
| Androgenetic alopecia | Female Middle aged-elderly | Diffuse alopecia that spares the frontal hairline | No | – | No | No | Progressive follicular miniaturization and nonspecific, superficial perivascular infiltrate |
| High hairline | Receding frontal hairline | No | – | No | No | Miniaturization or absence of the follicles without inflammation |
The characteristic finding in trichoscopy of FFA is a marked decrease in the number of follicular ostia in the central part of the area of alopecia, accompanied by erythema, with perifollicular scaling in the peripheral part of the plaque. In addition, the presence of branched capillaries, honeycomb pattern of pigmentation, patches and white dots, and vellus has been reported (Figure 1C and D).10 In 2012, Mireles-Rocha et al.45 described the presence of blue-grey dots surrounding some follicular units, a finding that until then had only been reported in LPP. The exact histopathological correlation of these trichoscopy findings is still not fully established, although it is accepted that the grey-whitish dots are usually observed in areas of greatest fibrosis, or those with greatest progression, whereas the grey-bluish dots are due to the presence of melanophages in the papillary dermis as a result of interface dermatitis and pigmentary incontinence observed in LPP and lupus erythematosus.44–46
For the histopathology study, it is important to bear in mind that biopsy will be of limited value in late stages, as the findings will be similar to other cicatricial alopecias.11 To obtain more specific histopathologic findings, it is necessary to study recent lesions in which signs of disease progression are observed. The biopsy should include hair follicles and perifollicular papules if they are present, and cross-sectional cuts of the sample should also be taken.20
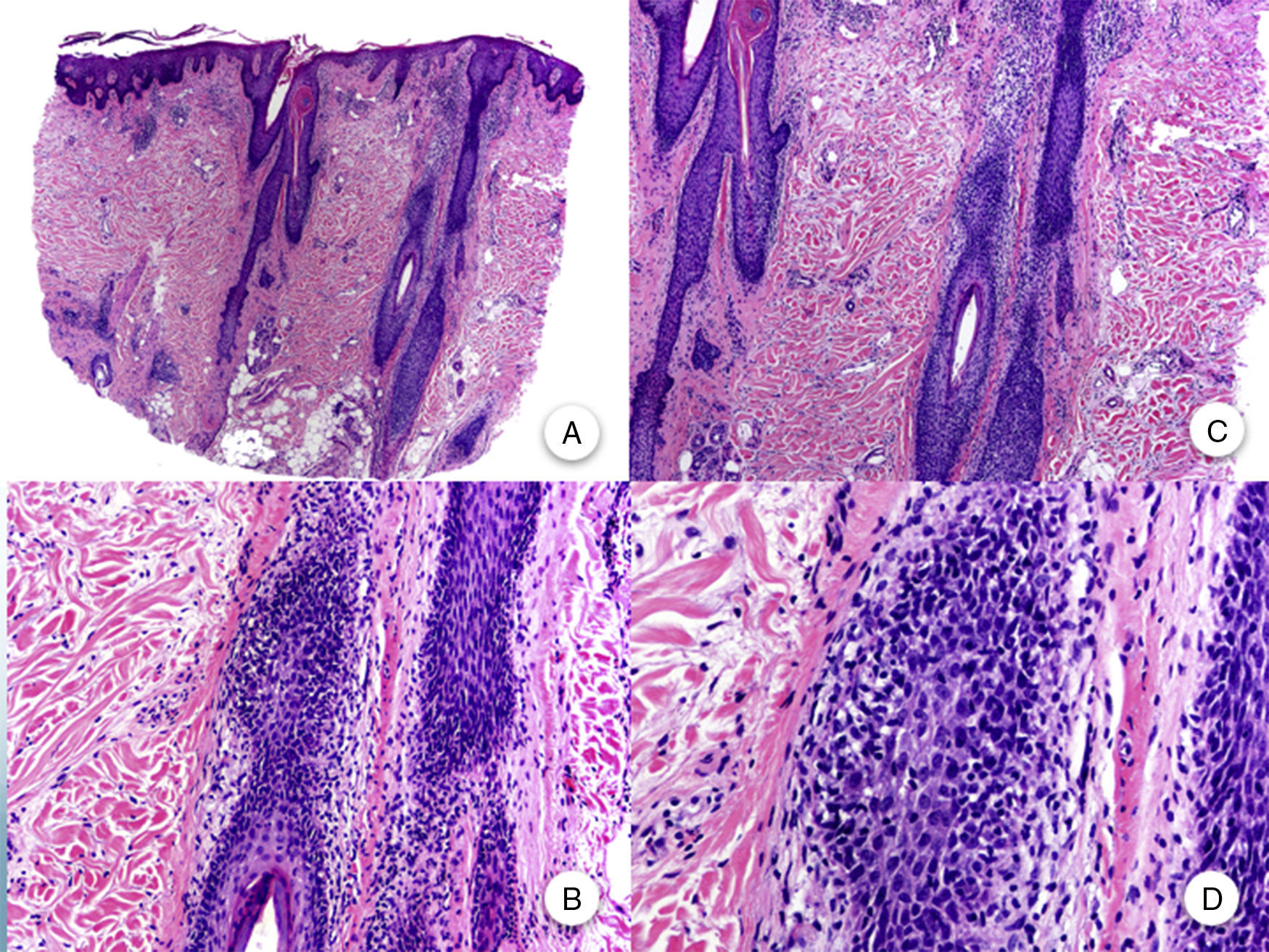
FFA is considered a primary lymphocytic cicatricial alopecia due to the presence of a lymphocytic inflammatory infiltrate of lichenoid appearance, which is preferentially located in the infundibulum and isthmus of the hair follicle and which does not involve the interfollicular epidermis.11,20,47 Although some articles emphasize the greater involvement of intermediate and vellus follicles,11 it is currently accepted that terminal follicles are just as affected as the aforementioned follicles.47 The inflammatory infiltrate is accompanied by vacuolar degeneration of the basal layer of the follicular epithelium and by prominent eosinophilic apoptosis of cells of the outer root sheath at the level of the infundibulum and isthmus.48 This region corresponds to the bulge, which harbors the pluripotent stem cells of the follicle, and so damage to this area leads to irreversible cicatricial alopecia.47 With follicular destruction, apoptotic remnants of the outer root sheath, hair stem, and cuticulum are trapped in the dermis, thus favoring a granulomatous foreign body reaction.47 Dilation and hypergranulosis of the follicular infundibulum are also observed. As the disease progresses, the inflammatory infiltrate disappears and the aforementioned changes lead to progressive perifollicular fibrosis with a lamellar onion-ring form. Thus, hair follicles are replaced by fibrous tracts which, clinically, correspond to areas of total alopecia and loss of follicular orifices (Figure 3).3,11 Despite the uniform paleness seen clinically in these areas,2 the absence of solar elastosis is significant from the histopathological point of view.11 Moreover, the lower part of the hair follicle, sweat glands, and subcutaneous tissue are preserved.3
Histopathology of frontal fibrosing alopecia. A and B, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, At higher magnification, concentric perifollicular fibrosis and peripheral lymphocytic infiltrate are observed (H-E ×200, H-E ×400, respectively).
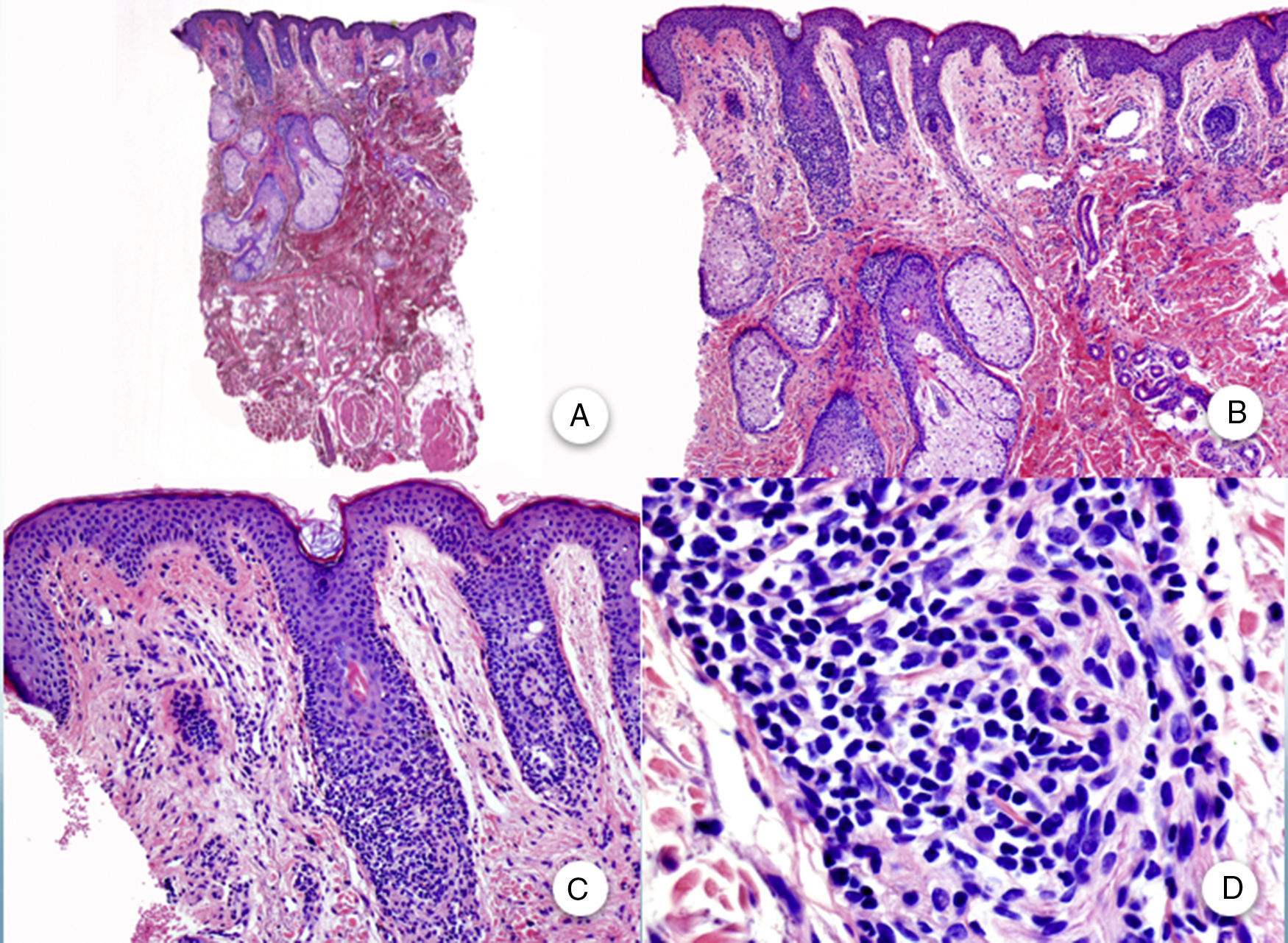
Similar histopathological findings can be observed in biopsies of the occipital region,3 eyebrows, and body hair. Although, clinically, these are noninflammatory and noncicatricial alopecias, inflammatory changes are observed in the remnant follicles in biopsy. In biopsies of the facial papules, similar changes are observed to those present in the scalp, but with involvement of the vellus type hair follicles (Figure 4).
Biopsy of facial papule. A and B, At low magnification, several vellus follicles can be observed with numerous sebaceous glands (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, Detail of the inflammatory infiltrate surrounding the upper portion of the vellus follicles (H-E ×200, HE ×400, respectively).
Although the clinical manifestations of FFA are different to LPP, the histopathological findings are similar in both entities, and so FFA is considered a clinical variant of LPP.2 Poblet et al.47 studied biopsy samples from both diseases to look for histopathological differences. The only differential findings were presence of perivascular infiltrate in the dermis in the case of LPP that was not present in FFA, interfollicular epidermal involvement in 50% of cases of LPP but not observed in FFA, and the presence of colloidal bodies or fibrosis in the papillary dermis in LPP but not FFA. In addition, less lichenoid tissue reaction was observed in FFA, fewer apoptotic cells in LPP, and, in some cases, substantial damage to the basal layer in LPP that was not present in FFA.
The immunohistochemical findings are also common to both diseases. Inflammatory lymphocytic infiltrate, composed of T lymphocytes with HLA-DR activation (LN3), a similar proportion of CD4 and CD8, and positive antibodies against dendrocytes (factor XIIIA) and macrophages (MAC387) are observed.3
Immunofluorescence can help in the differential diagnosis of LPP and FFA with chronic cutaneous lupus erythematosus (CCLE). In LPP, the abundant Civatte bodies are positive for IgM and less frequently for IgA, IgG, and C3, and they predominate in the follicular epithelium of the infundibulum and the isthmus.49 This finding, although a characteristic highly suggestive of LPP, is not pathognomonic, as it can also be observed in CCLE. However, it seems that the two processes can be differentiated according to the composition of these Civatte bodies, which are formed of necrotic keratinocytes (expressing cytokeratins) in LPP and of aggregates of the basement membrane (positive for collagen IV) in CCLE.49
Staining of elastic fibers can also help in differential diagnosis with other cicatricial alopecias, as a wedge-shaped scar with a larger base in the superficial dermis is present in LPP and FFA lesions, with destruction of elastic fibers only in this area, whereas in CCLE lesions, destruction of the perifollicular elastic fibers is observed.49–51 The elastic fibers are observed slightly thickened in pseudopelade of Brocq.
Laboratory abnormalities have not been reported in the published series. The results of the blood counts, biochemistry, liver function, thyroid hormones, and hepatitis C serology did not show any findings of interest. In addition, the levels of sex hormones, including luteinizing hormone, follicle-stimulating hormone, androgenous hormones, and prolactin were as expected for the age of the patients studied.2,3,10,11,17,19,47,52 Kossard et al.3 described a case of positivity for antinuclear antibodies at low titers, and Vañó-Galván et al.10 reported the presence of positive antithyroid antibodies and antinuclear antibodies in 10% and 7% of the patients studied, respectively, and so the authors recommend to rule out autoimmune thyroid disease in patients with FFA.
Prognosis and TreatmentNo clinical trials have been conducted to date to study the different treatment alternatives in FFA. The information available in the literature has been obtained from retrospective, observational studies that measure the effectiveness of treatments used according to objective outcomes such as the LPP activity index. This, combined with the frequent delay in diagnosis of the disease, the possibility of spontaneous stabilization in some patients, and the fact that scarring in areas of alopecia limits reversibility of the disease means that strong evidence is not available for an evidence-based treatment protocol for this disease.
The most frequently used drugs in studies published to date are numerous and varied, but in general, the use of corticosteroids applied topically or intralesionally,2,11,12,17 antimalarial agents,3,8,12,53 and 5-α-reductase inhibitors predominate.11,16–18Table 2 summarizes the different therapeutic approaches used to date, with the outcomes obtained in the studies published in the literature.
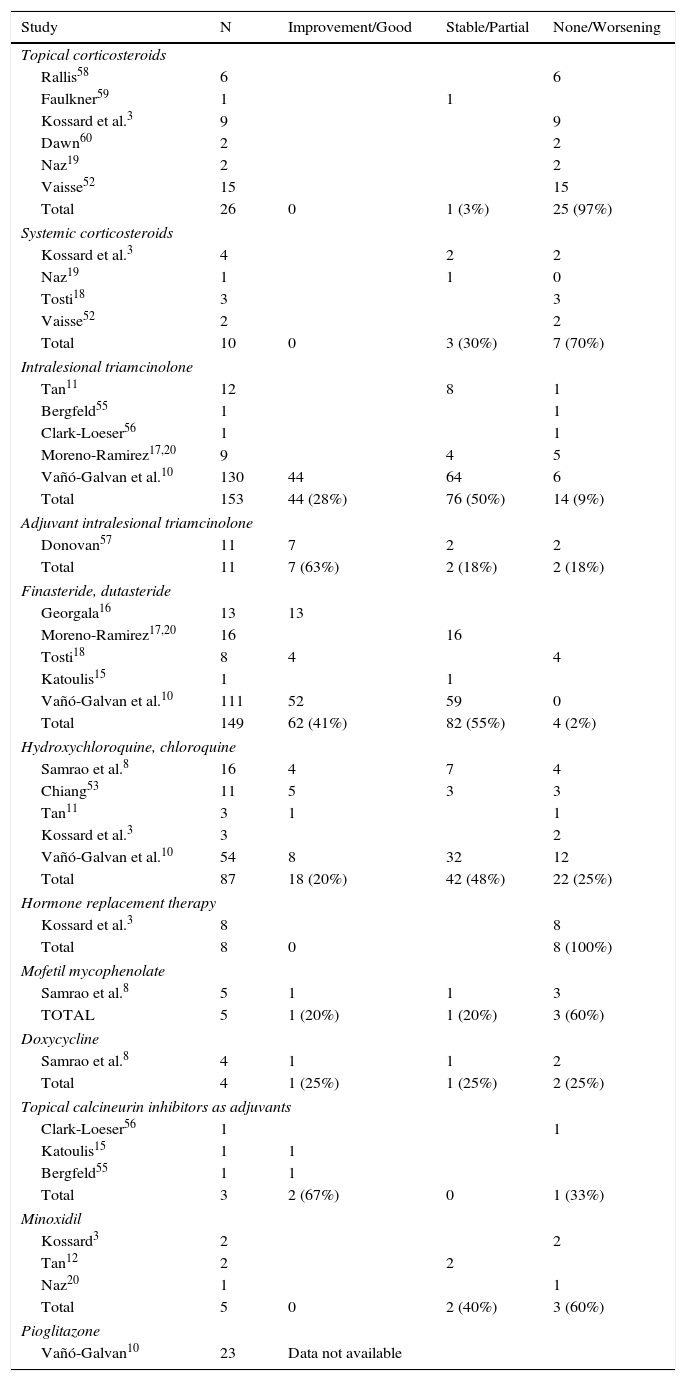
Response to the Different Treatments in the Published Studies.
| Study | N | Improvement/Good | Stable/Partial | None/Worsening |
|---|---|---|---|---|
| Topical corticosteroids | ||||
| Rallis58 | 6 | 6 | ||
| Faulkner59 | 1 | 1 | ||
| Kossard et al.3 | 9 | 9 | ||
| Dawn60 | 2 | 2 | ||
| Naz19 | 2 | 2 | ||
| Vaisse52 | 15 | 15 | ||
| Total | 26 | 0 | 1 (3%) | 25 (97%) |
| Systemic corticosteroids | ||||
| Kossard et al.3 | 4 | 2 | 2 | |
| Naz19 | 1 | 1 | 0 | |
| Tosti18 | 3 | 3 | ||
| Vaisse52 | 2 | 2 | ||
| Total | 10 | 0 | 3 (30%) | 7 (70%) |
| Intralesional triamcinolone | ||||
| Tan11 | 12 | 8 | 1 | |
| Bergfeld55 | 1 | 1 | ||
| Clark-Loeser56 | 1 | 1 | ||
| Moreno-Ramirez17,20 | 9 | 4 | 5 | |
| Vañó-Galvan et al.10 | 130 | 44 | 64 | 6 |
| Total | 153 | 44 (28%) | 76 (50%) | 14 (9%) |
| Adjuvant intralesional triamcinolone | ||||
| Donovan57 | 11 | 7 | 2 | 2 |
| Total | 11 | 7 (63%) | 2 (18%) | 2 (18%) |
| Finasteride, dutasteride | ||||
| Georgala16 | 13 | 13 | ||
| Moreno-Ramirez17,20 | 16 | 16 | ||
| Tosti18 | 8 | 4 | 4 | |
| Katoulis15 | 1 | 1 | ||
| Vañó-Galvan et al.10 | 111 | 52 | 59 | 0 |
| Total | 149 | 62 (41%) | 82 (55%) | 4 (2%) |
| Hydroxychloroquine, chloroquine | ||||
| Samrao et al.8 | 16 | 4 | 7 | 4 |
| Chiang53 | 11 | 5 | 3 | 3 |
| Tan11 | 3 | 1 | 1 | |
| Kossard et al.3 | 3 | 2 | ||
| Vañó-Galvan et al.10 | 54 | 8 | 32 | 12 |
| Total | 87 | 18 (20%) | 42 (48%) | 22 (25%) |
| Hormone replacement therapy | ||||
| Kossard et al.3 | 8 | 8 | ||
| Total | 8 | 0 | 8 (100%) | |
| Mofetil mycophenolate | ||||
| Samrao et al.8 | 5 | 1 | 1 | 3 |
| TOTAL | 5 | 1 (20%) | 1 (20%) | 3 (60%) |
| Doxycycline | ||||
| Samrao et al.8 | 4 | 1 | 1 | 2 |
| Total | 4 | 1 (25%) | 1 (25%) | 2 (25%) |
| Topical calcineurin inhibitors as adjuvants | ||||
| Clark-Loeser56 | 1 | 1 | ||
| Katoulis15 | 1 | 1 | ||
| Bergfeld55 | 1 | 1 | ||
| Total | 3 | 2 (67%) | 0 | 1 (33%) |
| Minoxidil | ||||
| Kossard3 | 2 | 2 | ||
| Tan12 | 2 | 2 | ||
| Naz20 | 1 | 1 | ||
| Total | 5 | 0 | 2 (40%) | 3 (60%) |
| Pioglitazone | ||||
| Vañó-Galvan10 | 23 | Data not available | ||
Corticosteroids are the first therapeutic strategy in LPP.2 The outcomes obtained depend mainly on the chosen route of administration. There is some debate in the literature regarding the use of intralesional corticosteroids. According to the most recent meta-analysis,54 the outcome varies from no response2,55,56 to partial response in 60% of patients, with no reports of full response.11,17 However, the most recently published study,10 with more patients than the meta-analysis, reported improvement in 33% of cases, with partial response/stabilization in 50% of patients. These discrepancies may be due to the timing of steroid application, as these drugs are only effective when inflammation is present in biopsy.10,17 In fact, in advanced cases of FFA, these drugs may have a detrimental effect on fibrosis and atrophy.20 Of note is that in the case of eyebrow alopecia, 80% of the patients respond partially or totally to treatment.57 Systemic corticosteroid administration, on the other hand, leads to disease stabilization in 43% of the patients,54 but progression of alopecia resumes on suspending treatment. Finally, corticosteroids administered topically2,19,52,58–60 or intramuscularly18 have not demonstrated any improvement in the disease in most of the studies published to date.
5-α-Reductase InhibitorsThe 5-α-reductase inhibitors, finasteride and dutasteride, have shown variable results, although in all cases described, at least a stabilization of FFA progression was achieved. Before 2007, the 5-α-reductase inhibitors had been used as coadjuvant treatment with other treatments, mainly intralesional corticosteroids and minoxidil, with disease stabilization rates of around 14% and an increased density of hair follicles.17 Subsequently, in studies of these agents as monotherapy 5-α-reductase inhibitors showed both stabilization and improvement in FFA, particularly in the case of dutasteride, which has a greater inhibitory activity because it acts on both isoenzymes of 5-α-reductase.13 The most recent study conducted in 355 patients reported improvement and stabilization of the disease in 57% and 53% of the cases, respectively.10 The best outcomes with these drugs have been obtained in studies that have included a high number of patients with AGA present in addition to FFA,10,17 and so it is likely that the high response rates are in part dependent on the accompanying AGA in these patients.
Oral Antimalarial AgentsChloroquine and hydroxychloroquine have demonstrated lower efficacy rates than previous drugs, but they are also an effective therapeutic alternative in the treatment of FFA. They have shown that they can stop disease progression in 48% of cases and improvements have been reported in 20% of patients in some series.8,10,53
Other DrugsTopical calcineurin inhibitors, such as tacrolimus and pimecrolimus, have been shown to be somewhat effective in the initial phases of the disease through their antiinflammatory effect, and with no danger of worsening the skin atrophy typically seen in FFA. However, as monotherapy, these drugs do not modify the course of the disease. Some studies have shown that administration as coadjuvants leads to an improvement in inflammatory symptoms in more than 50% of the patients.54
Cyclosporin is a drug used in LPP although its effects in FFA are unknown. It has been suggested that it may be effective due to the common histopathological substrate in the 2 diseases. To date, cyclosporin has only been used in 1 patient with FFA, and she had to withdraw from treatment due to adverse drug efects.8
Recently, pioglitazone (a PPAR-γ agonist) and rituximab (anti-CD20 antibody) have been proposed as possible treatment options in FFA given their selective action on proinflammatory molecules of the inflammatory lymphocytic infiltrate implicated in the pathogenesis of this disease. Ladizinski et al.13 used pioglitazone in a patient with FFA, but without any therapeutic response; however, in 2012, a study was published of its use in LPP, in which remission was observed in 20% of treated patients, with partial improvement in 50%. Vañó Galván et al.10 also performed a study with more than 23 patients treated with this drug, but the results have not yet been published.
Hormone replacement therapy,3 mofetil mycophenolate, doxycyline, topical monoxidyl,2,3,17–19,52 isotretinoin,3 griseofulvin,3 azathioprin,13 and interferon alfa13 have not been shown to be effective in any of the studies published to date. It has been observed that minoxidil increases capillary density in the treated area, but it does not modify the course of the disease.54 This drug is very useful when AGA and FFA are both present. Retinoic acid, used in topical monotherapy, was not shown to be effective in the treatment of FFA, although it can be useful for enhancing absorption of other topical agents, thus improving their efficacy. Oral retinoids, isotretinoin, and acitretin have been used at different doses with slight improvements in certain isolated cases.54
Hair transplantation has been used as a treatment for FFA, with studies such as those by Nusbaum et al.7 and Jiménez et al.40 confirming hair growth for 1.5-2years after transplantation, but also loss of more than 50% of transplanted hair within 3 years if no treatment is subsequently given. Most authors agree that hair transplantation is only recommended in cases in which there is a disease-free period that may range from 1 to 5 years,7,10,13 and provided that subsequent treatment is administered. Mendes-Bastos et al.61 proposed the use of 0.05% clobetasol propionate cream or topical calcineurin inhibitors along with systemic treatment with 5-α-reductase inhibitors and topical minoxidil for maintenance of transplanted hair.
In conclusion, to date, 5-α-reductase inhibitors have been shown to be the most effective drugs in the treatment of FFA, particularly when AGA is also present, and these agents are also useful as maintenance therapy. In the case of pruritus, erythema, and hyperkeratosis, intralesional corticosteroids may be indicated, with particular emphasis on eyebrow involvement, where the best outcomes have been obtained. Antimalarial agents and topical calcineurin inhibitors are an alternative as coadjuvant therapy. Finally, a wide range of cosmetic treatments are available, with eyebrow micropigmentation being of particular note.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that patient data do not appear in this article.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Esteban-Lucía L, Molina-Ruiz AM, Requena L. Actualización en alopecia frontal fibrosante. Actas Dermosifiliogr. 2017;108:293–304.

![Histopathology of frontal fibrosing alopecia. A and B, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, At higher magnification, concentric perifollicular fibrosis and peripheral lymphocytic infiltrate are observed (H-E ×200, H-E ×400, respectively). Histopathology of frontal fibrosing alopecia. A and B, Perifollicular infiltrate is observed in longitudinal sections (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, At higher magnification, concentric perifollicular fibrosis and peripheral lymphocytic infiltrate are observed (H-E ×200, H-E ×400, respectively).](https://static.elsevier.es/multimedia/15782190/0000010800000004/v1_201704280927/S1578219017300598/v1_201704280927/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Biopsy of facial papule. A and B, At low magnification, several vellus follicles can be observed with numerous sebaceous glands (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, Detail of the inflammatory infiltrate surrounding the upper portion of the vellus follicles (H-E ×200, HE ×400, respectively). Biopsy of facial papule. A and B, At low magnification, several vellus follicles can be observed with numerous sebaceous glands (hematoxylin-eosin [H-E] ×10, H-E ×100, respectively). C and D, Detail of the inflammatory infiltrate surrounding the upper portion of the vellus follicles (H-E ×200, HE ×400, respectively).](https://static.elsevier.es/multimedia/15782190/0000010800000004/v1_201704280927/S1578219017300598/v1_201704280927/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)


