Until quite recently, clinical guidelines and reviews on the treatment of hyperhidrosis advised against the use of systemic therapies based on their unacceptable adverse effects and a lack of evidence of usefulness. Numerous studies published over the past few years, however, have shown that, when used appropriately, these treatments are effective and in general have a favorable tolerability profile, making them an additional option for the treatment of hyperhidrosis, particularly for disease that is widespread, multifocal, or resistant to other treatments. In this review, the first of its kind, we examine the systemic therapies available for hyperhidrosis, including antihypertensives, psychoactive agents, and in particular oral anticholinergics, although none of these drugs are currently approved for this indication.
Hasta hace pocos años las guías clínicas y revisiones sobre tratamientos de la hiperhidrosis consideraban que no existía evidencia de la utilidad de los tratamientos sistémicos, y que se asociaban a un perfil intolerable de efectos adversos, siendo desaconsejados. Sin embargo, en los últimos años diferentes estudios han ido mostrando la eficacia de los mismos, asociándose a un perfil de efectos adversos por lo general aceptable cuando se usan de forma apropiada, convirtiéndose en una alternativa terapéutica más en el tratamiento de la hiperhidrosis, de especial relevancia en casos de hiperhidrosis generalizada, multifocal o resistente a otros tratamientos. Mediante esta revisión, la primera centrada en este tema, se repasarán los diferentes tratamientos sistémicos actualmente disponibles para la hiperhidrosis, incluyendo antihipertensivos, psicofármacos y, fundamentalmente, los anticolinérgicos orales, aunque ninguno tiene indicación aprobada en el tratamiento de la hiperhidrosis.
Hyperhidrosis, which refers to the excessive production of sweat, i.e., the production of more sweat than the body needs, affects an estimated 3% of the general population.1
A brief overview of the mechanisms involved in the production of sweat is provided to aid understanding of the various treatments available for hyperhidrosis. Sweat glands are activated by the sympathetic nervous system. The signals are transmitted from the “thermoregulation center” in the hypothalamus to the sweat glands through preganglionic and postganglioinic sympathetic nerves. Acetylcholine is a key neurotransmitter in these synapses, as it stimulates the nicotinic receptors located between the preganglionic and postganglionic fibers at the synapses and the muscarinic receptors (primarily muscarinic M3) in the sweat glands.1
Multiple treatments exist for hiperhidrosis, including topical antiperspirants (mainly aluminum salt solutions), topical anticholinergics (mainly glycopyrrolate), botulinum toxin, iontophoresis, sympathectomy, and ablative surgical techniques targeting the sweat gland tissue.1–7 There is, however, no consensus on what treatment strategies should be applied in the different types of hyperhidrosis. Whatever the case, individual treatment should be guided by the area of the body affected, the intensity of sweating and its impact on the patient's quality of life, response to previous treatments (effectiveness and tolerance), personal history (e.g., other diseases, age, regular medication), and of course, the cost and availability of treatments. It is generally advisable to start with the least aggressive and the least expensive options.
Despite the range of treatments available, however, optimal control of sweating is frequently not achieved due to poor response or tolerance, fear of adverse effects or complications, or simply the lack of availability or cost of certain treatments.
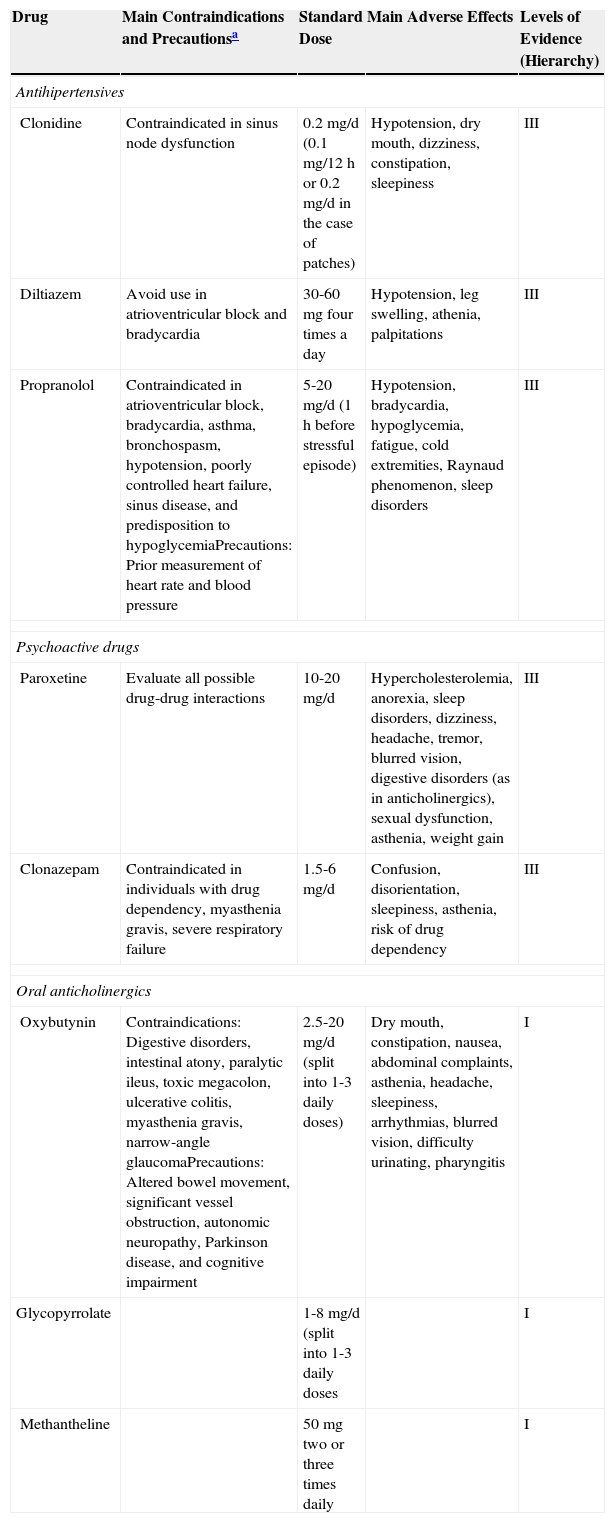
In this article, we focus on systemic rather than topical treatment of hyperhidrosis. Systemic treatments target the muscarinic receptors of sweat glands throughout the body, and there is therefore no risk of compensatory hyperhidrosis. These treatments tend to be cheap and associated with good patient adherence. Table 1 provides a summary of the systemic treatments available for hyperhidrosis, together with the corresponding levels of evidence based on the criteria in Table 2.8
Summary of Main Systemic Treatments Used in Hyperhidrosis.
| Drug | Main Contraindications and Precautionsa | Standard Dose | Main Adverse Effects | Levels of Evidence (Hierarchy) |
|---|---|---|---|---|
| Antihipertensives | ||||
| Clonidine | Contraindicated in sinus node dysfunction | 0.2mg/d (0.1mg/12h or 0.2mg/d in the case of patches) | Hypotension, dry mouth, dizziness, constipation, sleepiness | III |
| Diltiazem | Avoid use in atrioventricular block and bradycardia | 30-60mg four times a day | Hypotension, leg swelling, athenia, palpitations | III |
| Propranolol | Contraindicated in atrioventricular block, bradycardia, asthma, bronchospasm, hypotension, poorly controlled heart failure, sinus disease, and predisposition to hypoglycemiaPrecautions: Prior measurement of heart rate and blood pressure | 5-20mg/d (1h before stressful episode) | Hypotension, bradycardia, hypoglycemia, fatigue, cold extremities, Raynaud phenomenon, sleep disorders | III |
| Psychoactive drugs | ||||
| Paroxetine | Evaluate all possible drug-drug interactions | 10-20mg/d | Hypercholesterolemia, anorexia, sleep disorders, dizziness, headache, tremor, blurred vision, digestive disorders (as in anticholinergics), sexual dysfunction, asthenia, weight gain | III |
| Clonazepam | Contraindicated in individuals with drug dependency, myasthenia gravis, severe respiratory failure | 1.5-6mg/d | Confusion, disorientation, sleepiness, asthenia, risk of drug dependency | III |
| Oral anticholinergics | ||||
| Oxybutynin | Contraindications: Digestive disorders, intestinal atony, paralytic ileus, toxic megacolon, ulcerative colitis, myasthenia gravis, narrow-angle glaucomaPrecautions: Altered bowel movement, significant vessel obstruction, autonomic neuropathy, Parkinson disease, and cognitive impairment | 2.5-20mg/d (split into 1-3 daily doses) | Dry mouth, constipation, nausea, abdominal complaints, asthenia, headache, sleepiness, arrhythmias, blurred vision, difficulty urinating, pharyngitis | I |
| Glycopyrrolate | 1-8mg/d (split into 1-3 daily doses | I | ||
| Methantheline | 50mg two or three times daily | I | ||
Levels of Evidence Supporting Treatments.
| Hierarchical Level | Study Design | Bias |
|---|---|---|
| I | Systematic review and meta-analysis | + |
| I | Randomized clinical trials | ++ |
| II | Observational studies: cohort and case-control studies | +++ |
| III | Case reports and series | ++++ |
| IV | Expert opinions | +++++ |
aThis hierarchy is based on potential risk of bias, with studies offering the least risk ranked highest.
Systemic therapies targeting the cause. Systemic treatments that target the cause of excessive sweating are used to treat cases of secondary hyperhidrosis. An example would be hormone replacement therapy in the case of postmenopausal hyperhidrosis,2 but it should be noted that this option is not free of adverse effects.
We will focus on treatments that act on the mechanisms of sweat production, as most patients have primary rather than secondary hyperhidrosis. Signed informed consent must be obtained for each of the treatments described below, as they are not approved for use in hyperhidrosis.
Antihipertensives. The most widely used antihypertensives in hyperhidrosis are clonidine, diltiazem, and propranolol.
Clonidine is an α-adrenergic agonist that reduces sympathetic tone and increases the drive of the parasympathetic nervous system. It has been used in hyperhidrosis since 1984, even though its usefulness in this condition is supported by isolated experiences.9–13 In a recent study of 13 patients treated with clonidine, there were 6 responders and 7 treatment failures, including 3 nonresponders and 4 patients who developed hypotension,14 which is an adverse effect that needs to be considered with this drug. According to some authors, clonidine might be most useful in the treatment of craniofacial hyperhidrosis in postmenopausal women or women with flushing,13,14 although there have been isolated reports of clonidine patches being successfully used to treat gustatory facial sweating.11
Diltiazem is a calcium channel blocker approved for the treatment of mostly mild to moderate hypertension and certain arrhythmias; it is also used in the treatment and prevention of ischemic heart disease. There have been anectodal reports of good results in patients with hyperhidrosis treated with doses of between 30 and 60mg of diltiazem administered 4 times a day.15 The effect of this drug in hyperhidrosis has been attributed to the important role of calcium in the stimulation of sweat secretion.
Propranolol has been widely used in dermatology for some years now following demonstration of its value in the treatment of infantile hemangiomas.16 It is a β-blocker indicated for the treatment of hypertension, ischemic heart disease, and tachycardia. Its usefulness in hyperhidrosis is probably linked to its anxiolytic effect.1
Psychoactive drugs. Psychoactive drugs include antidepressants, antipsychotics, and anticonvulsants. Their use in hyperhidrosis is somewhat paradoxical considering that one of their possible adverse effects is excessive sweating. Their effectiveness in this condition might be due to the fact that they cause a certain indifference among patients to emotional triggers that frequently lead to sweating,1 but their anticholinergic and noradrenergic functions also probably have a role.17–19
The selective serotonin reuptake inhibitor paroxetine is used at a dose of 10 to 20mg/d.20,21
Of note in the group of benzodiazepines used to treat hypertrichosis is the antiepileptic drug clonazepam.22
Quetiapine17 and topiramate18,19 have also been reported to be effective in patients with hyperhidrosis.
Oral anticholinergics. Oral anticholinergics are the most widely used group of drugs in the systemic treatment of hyperhidrosis and as such will be discussed in more detail. They inhibit sympathetic activation by competing for acetylcholine receptors on sweat glands.
Their most common adverse effects occur in the gastrointestinal tract (mainly dry mouth and throat, although they can also cause constipation and even paralytic ileus), the eyes (mydriasis and cycloplegia, possibly leading to narrow-angle glaucoma), and the genitourinary tract (urinary frequency and even acute urinary retention). Central nervous system adverse effects (sleepiness, nervousness, headache, nausea, asthenia etc.) are relatively uncommon, as are cardiovascular effects such as tachycardia and palpitations.
Before prescribing an anticholinergic, thus, it is essential to obtain an accurate medical history, including personal history and current medications, and to inform patients of possible adverse effects. These drugs are absolutely or relatively contraindicated in individuals with urinary retention or with risk factors for this condition (e.g., patients with benign prostate hyperplasia), serious gastrointestinal disorders, such as inflammatory bowel disease, neuromuscular disorders, such as myasthenia gravis, and eye disorders, such as glaucoma.
Nevertheless, the doses of oral anticholinergics used in systemic hyperhidrosis treatment tend to be much lower than those used for their approved indications. Therefore, with the exception of dry mouth and throat, which are common, other adverse effects are rare or generally well tolerated.
In my opinion, women of childbearing age should be advised to use contraception while using oral anticholinergics (due to insufficient data on their safety in pregnancy) and also to avoid alcohol (due to the potentiating effects of anticholinergics), and to maintain a strict oral hygiene routine, as dry mouth can favor the development of caries.
Oral anticholinergics used in the systemic treatment of hyperhidrosis include methantheline,23,24 glycopyrrolate,25–30 oxybutynin,31–55 propantheline,56 tolterodine,1 and solifenacin.1 In this article we will focus on the first 3 anticholinergics, as they have been studied most in recent years and are also associated with few adverse effects due to their limited passage through the blood-brain barrier. It should be noted that a lack of response or tolerance to one anticholinergic does not necessarily mean that another one will not be useful.
Methantheline bromide is approved as an adjuvant therapy for peptic ulcer due to its antisecretory effects and is also indicated for the treatment of irritable bladder in patients aged over 12 years. It is not available in Spain. Its use in the form of 50-mg tablets has been investigated in 2 double-blind, placebo-controlled, randomized clinical trials conducted in adults with focal hyperhidrosis.23,24 In the first trial,23 the patients were administered a single tablet twice a day, and in the second, more recent and larger trial, involving 339 patients, the patients were administered a tablet every 8hours.24 A moderate improvement was seen in both trials, but almost exclusively for axillary hyperhidrosis.It has been postulated that methantheline bromide is probably not useful for treating palmar hyperhidrosis, as this drug is excreted through sweat glands present in axillary but not palmoplantar sites. The adverse effects, which mostly consisted of dry mouth, were mild and well tolerated.
Glycopyrrolate (glycopyrronium bromide) is approved as a preoperative agent to reduce gastric secretions in gastric surgery, as an adjunct for peptic ulcer, and as an inhibitor of drooling in patients older than 12 years. It is sold in tablet form (1- or 2-mg tablets), although in Spain it is only available as a “foreign medication” in the 1-mg form. It costs approximately €150 for a hundred 1-mg tablets. Assuming an average dose of 3mg a day, a year's treatment would cost around €1600.
The use of glycopyrrolate in the systemic treatment of hyperhidrosis is supported by numerous reports dating back to 2007,13,25–30 although the usefulness of topical glycopyrrolate in hyperhidrosis (and craniofacial hyperhidrosis in particular) had been previously reported.57–63 The first study involved 24 patients: 9 patients with widespread hyperhidrosis and 15 with focal hyperhidrosis.25 The drug was administered in incremental doses based on response, with an initial dose of 2mg/12h (4mg/d) and a maximum dose of 4mg/12h (8mg/d). Response was evaluated in 19 of the 24 patients. There were 14 responders and an equal number of patients who developed adverse effects, mostly dry mouth. Of the 14 responders, 5 stopped treatment due to the adverse effects and 4 stopped due to a lack of effect. In another more recent study in which glycopyrrolate was administered to 45 patients at the standard dose of 1 to 4mg/d (1-2mg in most cases), there were 30 responders (66.6%).14 Of the 15 failures, 9 were due to limiting adverse effects and 6 to a lack of response.
A Korean group recently described its experience with glycopyrrolate administered at incremental doses with an initial dose of 2mg/d and a maximum dose of 8mg/d.26,27 In the first study, which involved 36 patients with primary hyperhidrosis, a significant improvement was observed in 75% of the patients, and adverse effects were reported in 36%.26 In the second study, which investigated the use of glycopyrrolate in 19 patients with compensatory hyperhidrosis following sympathectomy, 79% of patients responded well and 42% experienced adverse effects.27 None of the patients in either study stopped treatment because of adverse effects (mainly dry mouth). There has also been an isolated report of the successful use of glycopyrrolate in antidepressant-associated hyperhidrosis.29
Furthermore, while glycopyrrolate is not approved for use in patients younger than 12 years, Paller et al.8 published a study describing its use in 31 children and adolescents aged between 9 and 18 years with hyperhidrosis. Starting doses of 1 to 2mg/d were increased progressively to a maximum of 3mg/12h depending on response and tolerance. The vast majority of patients (90%) responded well, and adverse effects (again, mostly dry mouth) were observed in 29%. Similar results were reported in a more recent study of patients aged between 11 and 17 years treated with doses of 0.5 to 3mg/d.30
Oxybutynin hydrochloride is the oral anticholinergic with which the most experience has been acquired in hyperhidrosis. It is indicated for the treatment of the symptoms of urinary urgency and frequency in patients older than 5 years, although it is commonly used in even younger children. Its value in the treatment of hyperhidrosis was discovered by chance in 1988, when it was found to improve hyperhidrosis and hypothermia in a patient treated with oxybutynin to relieve urinary urgency.31 Its good results in hyperhidrosis were not described again until 1996, when, again by chance, it was found to improve excessive sweating in a patient with urinary urgency.32
Since then, numerous studies33–55 have reported its value as a systemic treatment for multifocal hyperhidrosis,31,33–38 focal hyperhidrosis,39–48 compensatory hyperhidrosis (or nonresponders) following sympathectomy,33,49,50 postmenopausal hyperhidrosis,32,51 and hyperhidrosis secondary to tricyclic antidepressants50 and selectrive serotonin reuptake inhibitors.35,53 It has not proven effective in improving exercise-induced (physiological) sweating.54,55
Most recent studies on the use of oxybutinin in hyperhidrosis have been published by Wolosker et al., who have documented its use in over 500 adults37–43,45–48 and 45 children.46 Wolosker and his team use incremental doses to enhance tolerance, with initial doses of 2.5mg/d and maximum doses of 10mg/d administered twice daily, except in children weighing less than 40kg, who are administered 5mg/d split into 2 doses. Other authors recommend starting at a dose of 1.25 mg/d, and increasing this progressively until the minimum effective dose is reached (with a maximum of 7.5mg/d split into 3 doses).36 In any case, the doses used in the majority of patients with hyperhidrosis reported in the literature are considered low, as according to the summary of product characteristics for oxybutynin, the recommended dose is up to 15 mg/d for adults and 10mg/d for children. As with other oral anticholinergics, the most common adverse effect seen with oxybutynin is dry mouth and there have been no reports of serious or irreversible effects. Another major advantage of oxybutynin is its price. Assuming a treatment regimen of 7.5mg/d, the annual cost of oxybutynin therapy is approximately €36.
Most studies of systemic hyperhidrosis treatment have several limitations, such as considerable losses to follow-up (possibly including many treatment failures due to nonresponse or adverse effects), short follow-up times (generally in the range of several weeks), and failure to determine optimal doses for individual cases.
Nevertheless, in my opinion, systemic hyperhidrosis therapy could be contemplated as first-line or second-line treatment (after antiperspirants) for widespread, multifocal, or compensatory hyperhidrosis; as second-line treatment (again, after antiperspirants) for craniofacial hyperhidrosis, (although topical anticholinergics could also be considered); and as third-line treatment for palmoplantar hyperhidrosis (after antiperspirants and iontophoresis) and axillary hyperhidrosis (after antiperspirants and botulinum toxin) (Fig. 1). The above treatments can also be combined in order to achieve better control of excessive sweating in certain areas of the body or in certain circumstances.
In my opinion, oxybutynin would currently be the first-choice systemic treatment for hyperhidrosis due to the greater experience with this drug and its availability (it is not a foreign medicine) and price. The second-choice would be oral glycopyrrolate. Antihypertensives should be reserved for patients with associated hypertension, propranolol for patients with anxiety symptoms (tremor, tachycardia), and chlonidine for patients with flushing. Optimal doses should be chosen on a case-by-case basis according to response and tolerance, and doses should always be increased progressively. Anxiolytics could be contemplated in patients with stress-associated hyperhidrosis, and antidepressants in patients with associated depressive disorders that might be triggering or exacerbating the patient's sweating.
In conclusion, there is sufficient evidence to support the use of systemic therapy for hyperhidrosis, particularly in the case of oral anticholinergics. These treatments offer numerous advantages, including versatility (potentially useful for all types and locations of hyperhidrosis), low price (in most cases), ease of follow-up, and lack of local irritation and compensatory hyperhydrosis. The above factors all favor good treatment adherence. Disadvantages include off-label use and frequent adverse effects (mainly dry mouth), although most effects are generally mild and well tolerated. Larger, randomized, placebo-controlled studies and longer follow-up periods are needed.
Conflicts of InterestThe author declares that he has no conflicts of interest.
Please cite this article as: del Boz J. Tratamiento sistémico de la hiperhidrosis. Actas Dermosifiliogr. 2015;106:271–277.